The impact of direct oral anticoagulants on viscoelastic testing – A systematic review
- 1Institute of Anesthesiology, University and University Hospital Zurich, Zurich, Switzerland
- 2Department of Outcomes Research, Anesthesiology Institute, Cleveland Clinic, Cleveland, OH, United States
Background: In case of bleeding patients and in acute care, the assessment of residual direct oral anticoagulant (DOAC) activity is essential for evaluating the potential impact on hemostasis, especially when a timely decision on urgent surgery or intervention is required. Viscoelastic tests are crucial in a modern goal-directed coagulation management to assess patients’ coagulation status. However, the role of viscoelastic test to detect and quantify residual DOAC plasma levels is controversially discussed. The aim of this review was to systematically summarize the evidence of viscoelastic tests for the assessment of residual DOAC activity.
Method: PubMed, Embase, Scopus, and the Cochrane Library were searched for original articles investigating the effect of rivaroxaban, apixaban, edoxaban, or dabigatran plasma levels on different viscoelastic tests of the adult population from database inception to December 31, 2021.
Results: We included 53 studies from which 31 assessed rivaroxaban, 22 apixaban, six edoxaban, and 29 dabigatran. The performance of viscoelastic tests varied across DOACs and assays. DOAC specific assays are more sensitive than unspecific assays. The plasma concentration of rivaroxaban and dabigatran correlates strongly with the ROTEM EXTEM, ClotPro RVV-test or ECA-test clotting time (CT) and TEG 6s anti-factor Xa (AFXa) or direct thrombin inhibitor (DTI) channel reaction time (R). Results of clotting time (CT) and reaction time (R) within the normal range do not reliable exclude relevant residual DOAC plasma levels limiting the clinical utility of viscoelastic assays in this context.
Conclusion: Viscoelastic test assays can provide fast and essential point-of-care information regarding DOAC activity, especially DOAC specific assays. The identification and quantification of residual DOAC plasma concentration with DOAC unspecific viscoelastic assays are not sensitive enough, compared to recommended anti-Xa activity laboratory measurements.
Systematic review registration: [https://www.crd.york.ac.uk/prospero/display_record.php?RecordID=320629], identifier [CRD42022320629].
Introduction
Direct oral anticoagulants (DOACs) are prescribed for stroke prevention in atrial fibrillation, for the prevention and treatment of venous thromboembolism and for secondary cardiovascular prevention (1, 2). Currently, five substances are approved with regional differences for clinical use by medical regulatory authorities: rivaroxaban, apixaban, edoxaban, and dabigatran (3). The prescription and use of DOACs is steadily increasing since the introduction in 2008 (4). They are taken orally as fixed-dose regimens with no regular monitoring required (5).
In acute care, the assessment of residual DOAC activity is essential for evaluating the potential impact on hemostasis, especially when a timely decision on urgent surgery or intervention is required (6–9). Residual DOAC plasma levels can be quantified by high-pressure liquid chromatography-tandem mass spectrometry (HPLC-MS) or by chromogenic anti-Xa and anti-IIa assays (10). However, both measurements are more time-consuming compared to point-of-care assays. HPLC-MS measurement requires on average 2 h, whereas specific DOAC anti-Xa assays deliver results within 30 min. No standardized point-of-care test is currently available to evaluate the anticoagulant effects of DOACs (7, 8). Viscoelastic tests are crucial in a modern-goal directed coagulation management to assess patients’ coagulation status (11, 12). The role of viscoelastic test to detect residual DOAC plasma levels in acute care is controversially discussed. Therefore, this systematic review compiles the existing evidence on the accuracy of point-of-care viscoelastic tests to assess residual DOAC effects.
Viscoelastic assays
Different from standard coagulation assays, viscoelastic tests are point-of-care systems analyzing in whole blood the process of clot formation and subsequent lysis in real time with on-line graphic display. The rotational thrombelastic system (ROTEM, Werfen, Bedford, MA, USA) and thrombelastographic system (TEG, Haemonetics Corporation, Boston, MA, USA) provide similar information but operate with different techniques. While ROTEM delta uses a rotating pin, the TEG 5000 uses a cup oscillating around the pin. The new ROTEM sigma operates by the same principle as delta but is automated with ready-to-use cartridges for simultaneous testing. The new TEG 6s system uses a resonance method, is fully automated and uses prefabricated cartridges, too. Differences in the terminology of the results between both devices are shown in Figure 1. ClotPro (Haemonetics Corporation, Boston MA, USA; formerly enicor GmbH, Munich, Germany) provides six channels for parallel testing. It has a unique Active-Tip technology with the dried reagents contained in a sponge at the pipette tip.
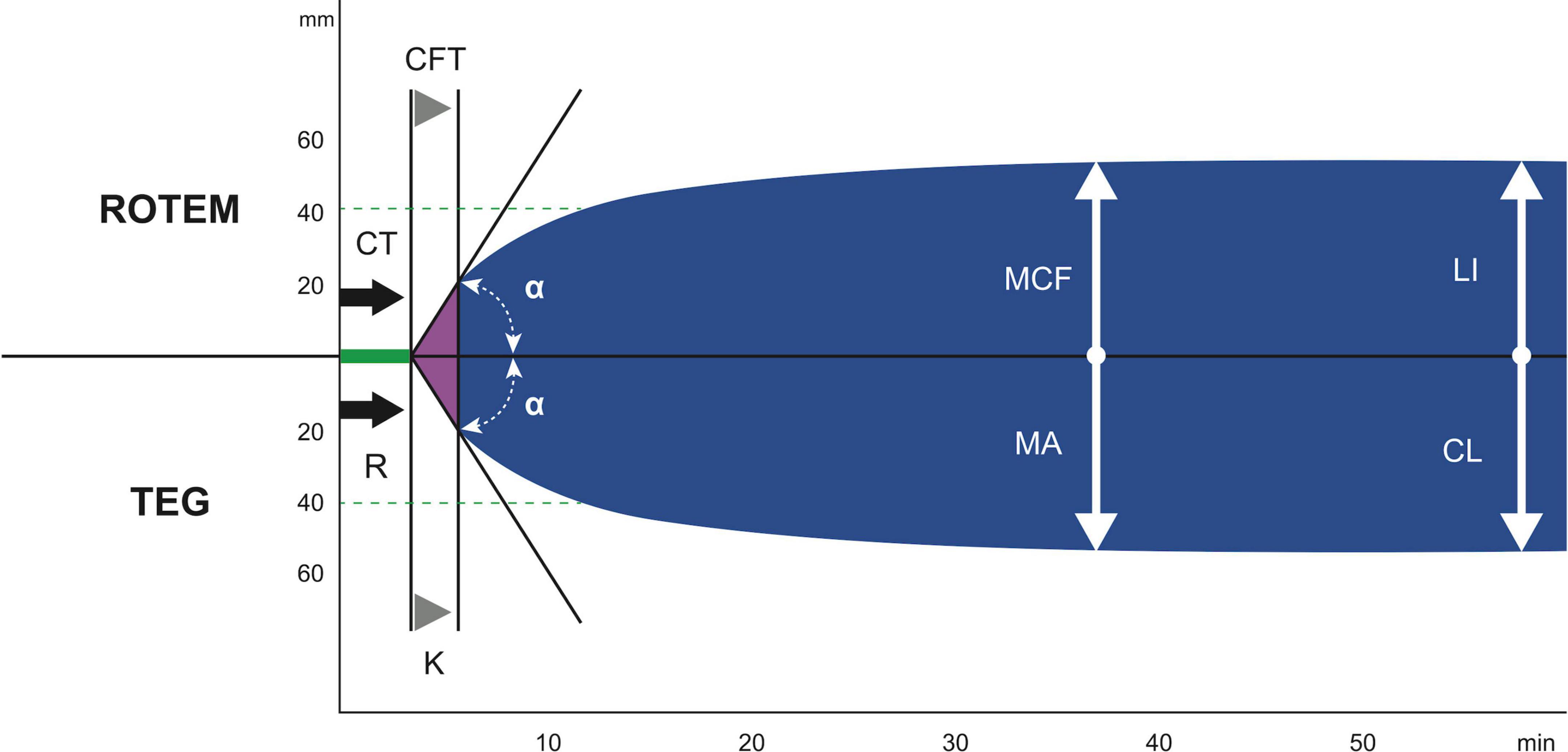
Figure 1. The difference in the terminology of the most important results between ROTEM and TEG. ROTEM parameters: CT, clotting time; CFT, clot formation time; α, α angle; MCF, maximum clot firmness; LI (30/60), lysis index 30 and 60 min after CT. TEG parameters: R, reaction time; k, kinetics; α, α angle; MA, maximum amplitude; CL (30/60), clot lysis after 30 and 60 min (89). With reprint permission by Georg Thieme Verlag KG.
With the different viscoelastic systems, several assays can be performed depending on the clinical question (13). For ROTEM and ClotPro, the clotting time (CT) and for TEG the reaction time (R) is defined as the time from the beginning of the test until a clot firmness amplitude of 2 mm is achieved which reflects the velocity of thrombin generation.
Methods
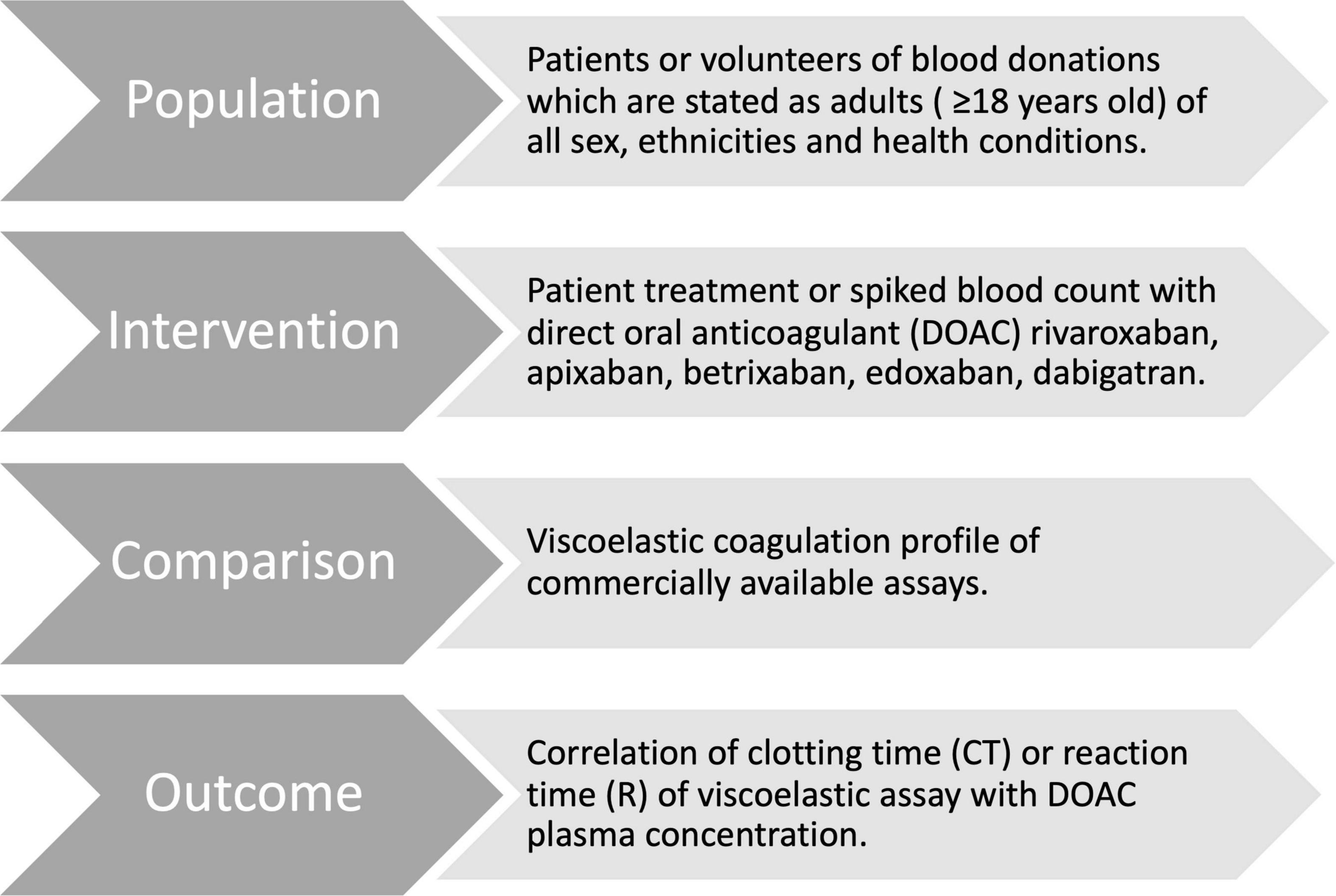
This systematic review follows the guidelines of PRISMA (Preferred Reporting Items for Systematic Reviews and Meta-Analyses) (Figure 2). We defined the PICO question for this review as “In adult patients taking DOACs (Population), are results in point-of-care viscoelastic tests (Intervention) compared to standardized laboratory tests or DOAC naïve blood (Control) altered by the drug (Outcome)?” This work was registered on the international prospective register of systematic reviews PROSPERO (registration ID # CRD42022320629).
Eligibility criteria and study selection
We included original articles addressing the coagulation profile of DOACs assessed with viscoelastic assays of the adult population (>18 years old) from database inception to December 31, 2021. Articles were excluded if they did not consider apixaban, edoxaban, rivaroxaban, and dabigatran; did not involve whole blood viscoelastic assays; instruments for measuring activated clotting time (ACT) solely; or referred to animal studies. Non-commercially available assays were considered beyond the scope of this review and are mentioned, but not further described. Poster abstracts and case reports were excluded too.
Search strategy
Four electronic databases have been queried: US National Library of Medicine (MEDLINE via PubMed), Excerpta Medica Database (EMBASE), Scopus database by Elsevier, and the Cochrane Library for Trials. We used following keywords and operators: (ROTEM or TEG or sonoclot or clotpro or reorox or viscoelastic or “viscoelastic hemostatic assay” or “viscoelastic test” or thrombelastometry or thrombelastography or thrombelastography or “hemostatic assay”) AND (DOAC or “direct oral anticoagulant” or NOAC or “new oral anticoagulants” or “non-vitamin k anticoagulants” or rivaroxaban or dabigatran or apixaban or edoxaban).
References of articles were retrieved for the inclusion of related articles. Publications in English and German language were considered.
Selection process
Two reviewers (CC and SDS) examined studies independently by reading the titles and abstracts. The studies corresponding to the inclusion criteria were read and the reviewers abstracted data. Any discrepancies between the reviewers were resolved by discussion.
Data items
A standard form was used to extract the following data: author(s), year of publication, study site and country, study design, number of overall patients, anticoagulant(s) examined, viscoelastic test(s) used, plasma concentrations of anticoagulant(s), main findings.
Risk of bias
The methodological quality of each included article was evaluated by the Newcastle Ottawa Scale (NOS) (14) (Supplementary Table 1). Three independent authors performed the evaluation (SDS, CC, and TRR). Disagreement was solved by discussion.
Statistics
We labeled the strength of the association, for absolute values of r, the following: 0 to 0.19 is regarded as very weak, 0.2 to 0.39 as weak, 0.40 to 0.59 as moderate, 0.6 to 0.79 as strong and 0.8 to 1 as very strong correlation (15). Regression analyses values of R2 were converted into an effect size f according to Cohen (16), where f = 0.10 represents a weak effect, f = 0.25 a moderate effect and f = 0.40 a strong effect (17).
Results
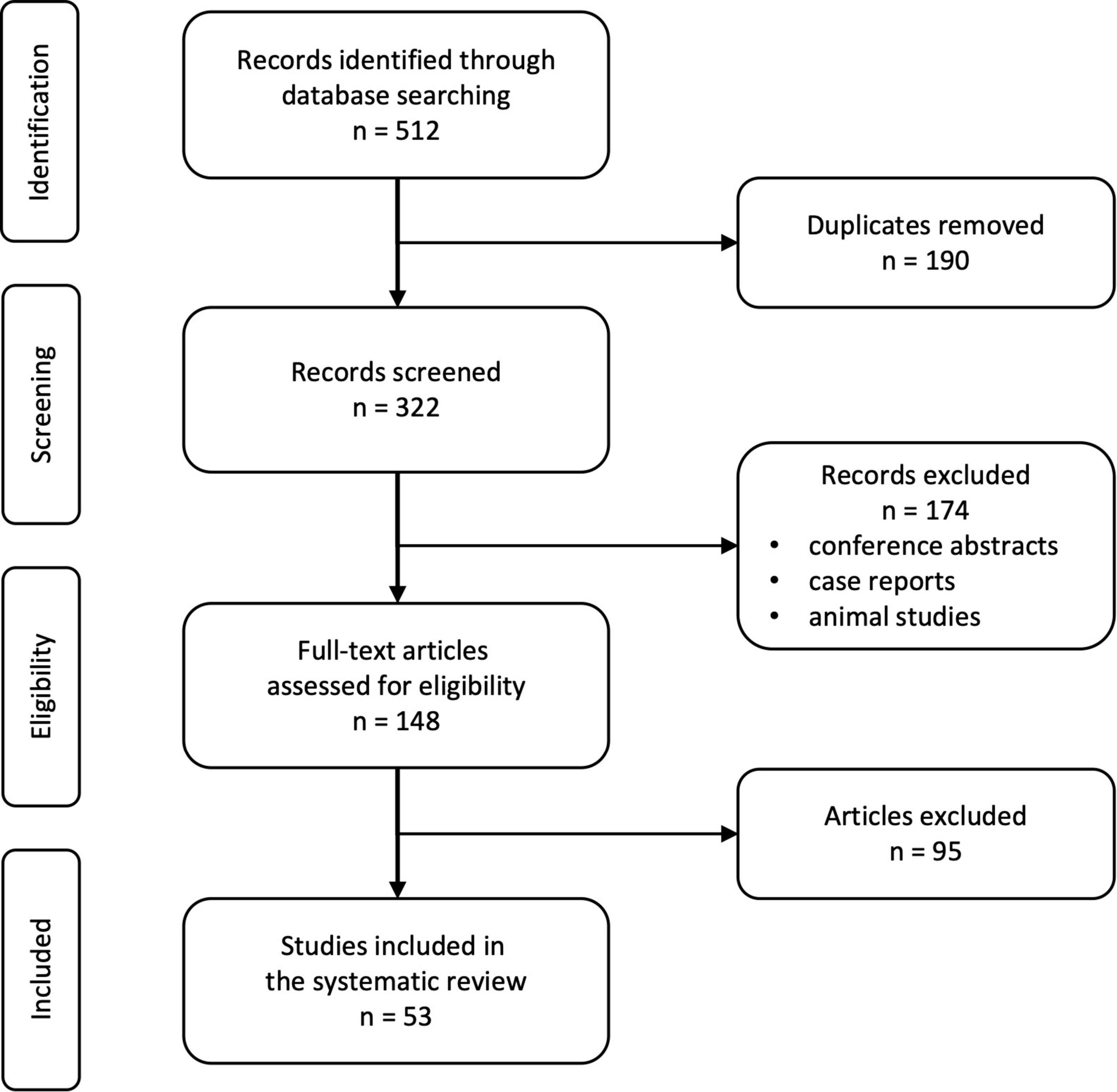
We identified 512 records in the mentioned databases of which 190 duplicates were automatically removed (Figure 3). The remaining 322 records were screened for suitability and a total of 148 full-text articles were proofread. A total of 53 studies met the pre-defined quality and inclusion criteria.
Viscoelastic analysis of direct factor Xa inhibitors
Rivaroxaban
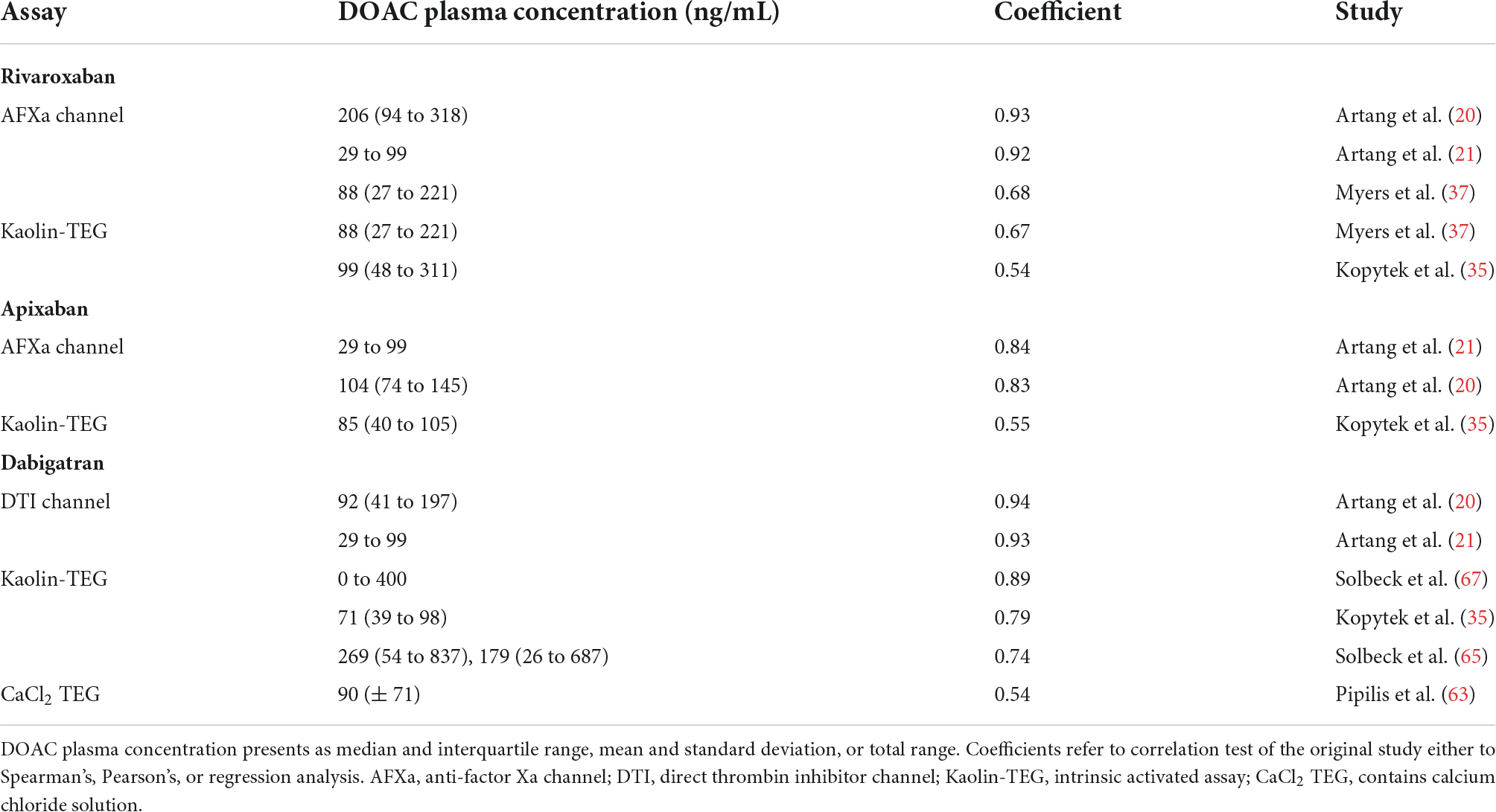
We identified 31 studies describing rivaroxaban action in whole blood with viscoelastic methods (18–48) (Supplementary Tables 2.1–2.3). Except for the well-documented viscoelastic parameters clotting time (CT) and reaction time (R), rivaroxaban showed either no significant influence or was not analyzed for other parameters besides individual and heterogenous nominations. The CT and R in relation to the rivaroxaban plasma concentration are shown in Figure 4.
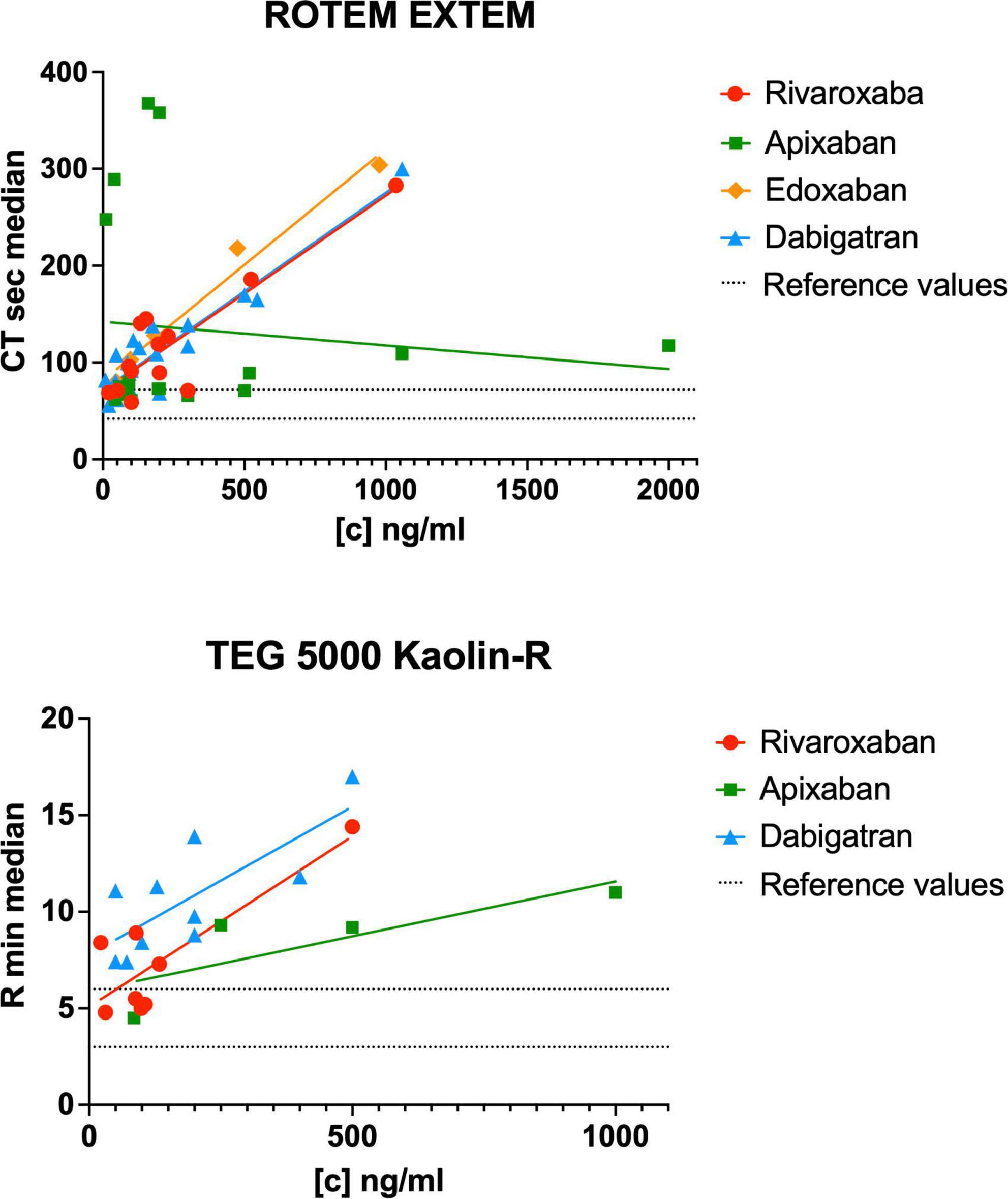
Figure 4. Reported clotting time (CT) and reaction time (R) in relation to the DOAC plasma concentration of the included studies for the ROTEM EXTEM and TEG 5000 kaolin assays. [c] DOAC plasma concentration in ng/ml. The dashed lines represent the lower and upper reference values for ROTEM EXTEM CT [42, 72 s (88)] and TEG 5000 R [3, 6 min (90)].
Rivaroxaban and rotational thrombelastometry
Seventeen studies assessed rivaroxaban measurements in whole blood with ROTEM (Werfen, Bedford, MA, USA) (18, 19, 23, 24, 27, 28, 30, 31, 34, 36, 39–41, 44, 45, 47, 48) (Supplementary Table 2.1). A significant correlation between rivaroxaban plasma concentration and duration of clotting time (CT) was shown for EXTEM (strong to very strong correlation) (24, 28, 30, 34, 44, 45), INTEM (moderate to strong correlation) (24, 28, 30, 34, 45), NATEM (strong correlation) (19), FIBTEM (strong correlation) (44), and HEPTEM (strong correlation) (28) assays (Table 1.1). One study did not find any significant effect of rivaroxaban on ROTEM parameters (31).
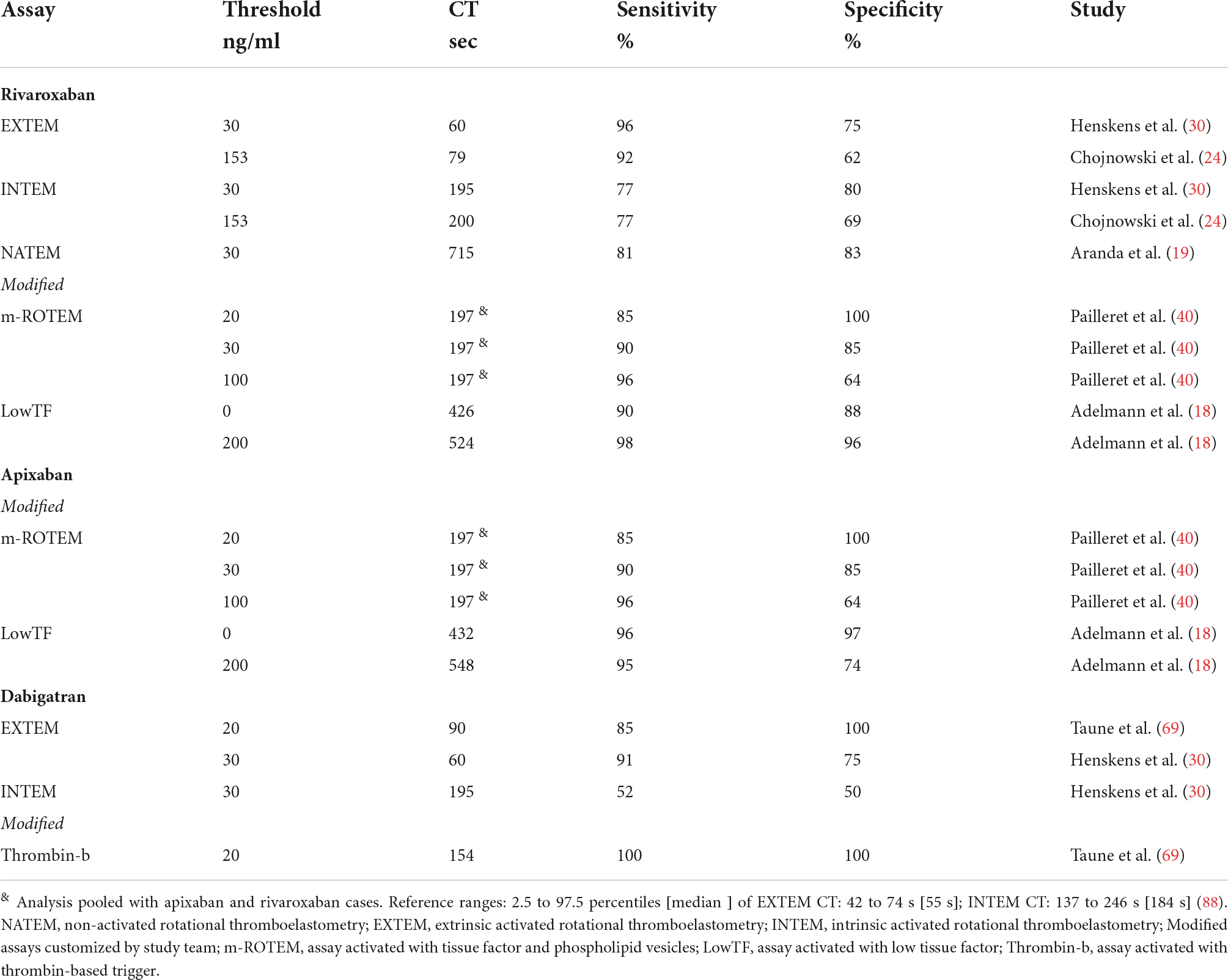
Table 1.1. Study defined ROTEM clotting time (CT) thresholds for detection of the residual DOAC plasma concentration stratified by assays.
Three studies examined modified ROTEM assays (18, 40, 48). Some of these non-commercially available modified ROTEM activators showed promising results in detecting rivaroxaban plasma levels (Table 2.1).
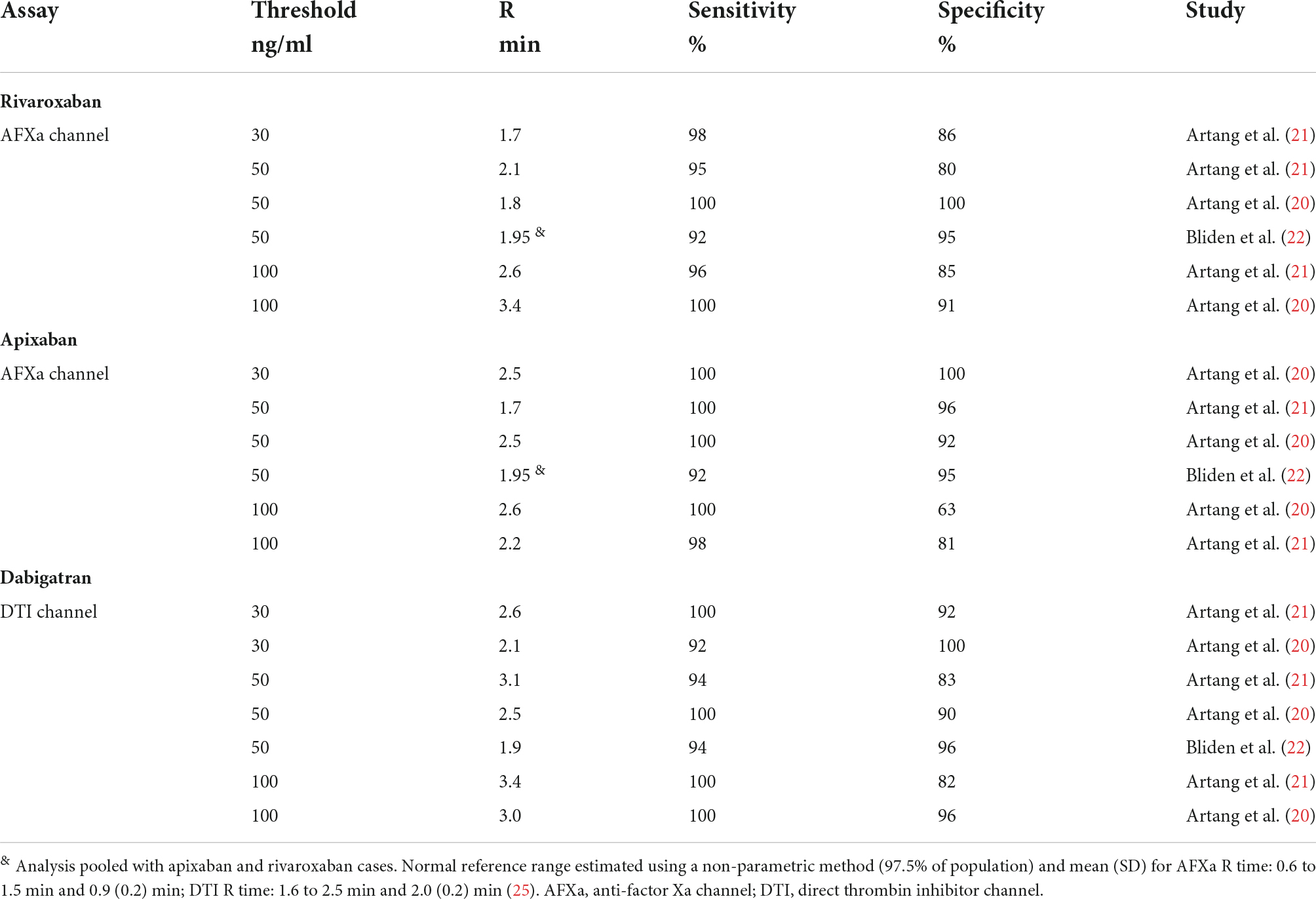
Table 1.2. Study defined TEG 6s reaction time (R) thresholds for detection of residual DOAC plasma concentration stratified by assays.
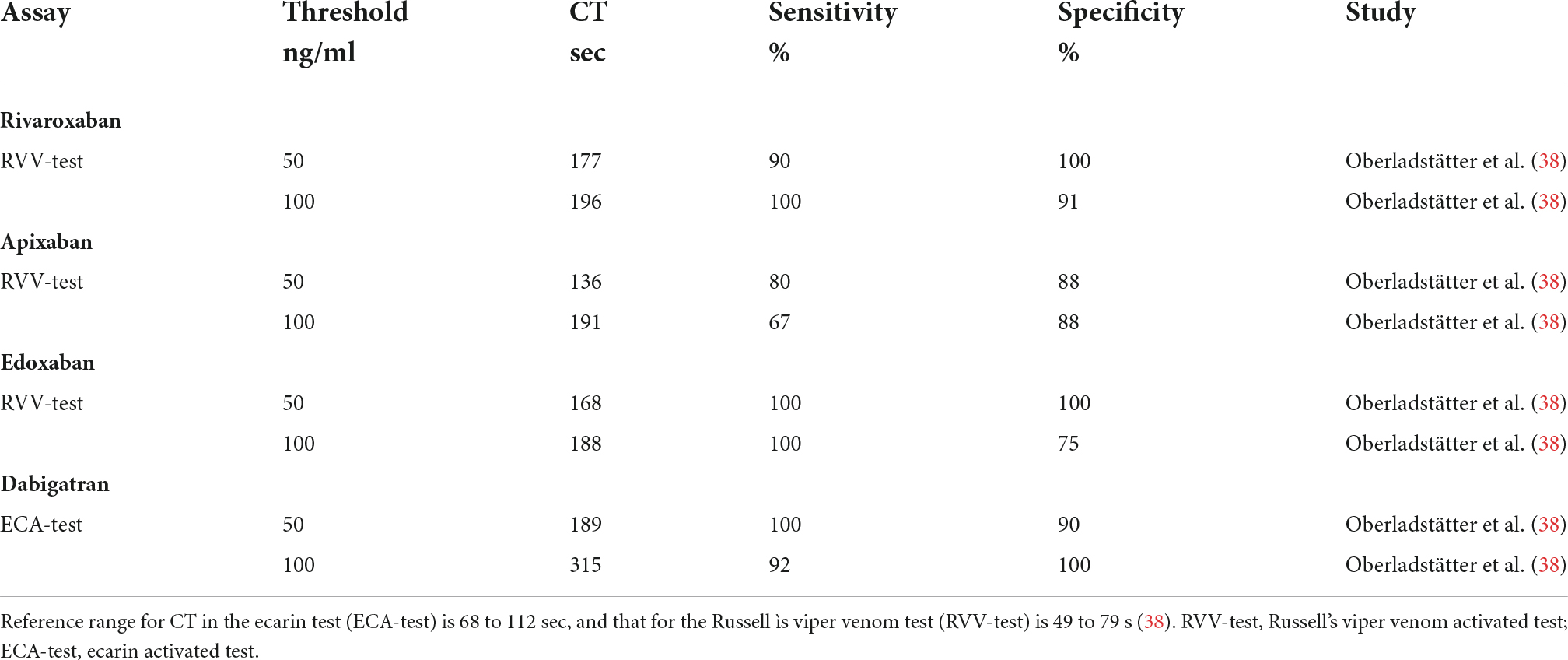
Table 1.3. Study defined ClotPro clotting time (CT) thresholds for detection of residual DOAC plasma concentration stratified by assays.
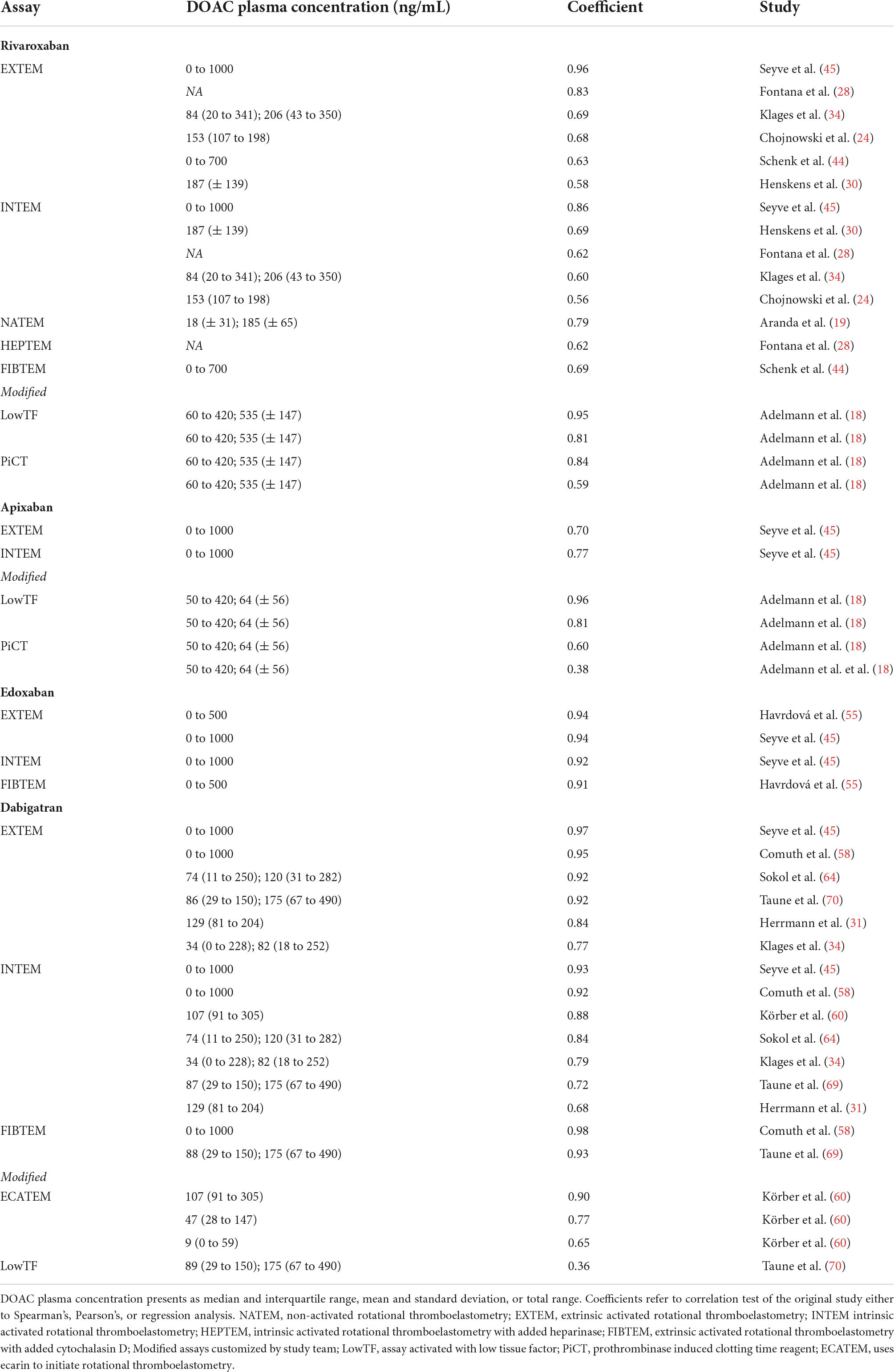
Table 2.1. Correlation of DOAC plasma concentration and ROTEM clotting time (CT) for different assays.
Rivaroxaban and thrombelastography
Thirteen studies described rivaroxaban action in whole blood with TEG (Haemonetics Corporation, Boston MA, U.S.A.) (20–22, 25, 26, 31–33, 35, 37, 42, 43, 46) (Supplementary Table 2.2). Of these, four studies did not report the exact rivaroxaban concentration under which conditions the viscoelastic tests were performed (22, 25, 32, 33). Four studies investigated with the new cartridge-based anti-factor-Xa [AFXa] channel of TEG 6s (20–22, 25), to the best of our knowledge not yet commercially available at the time of manuscript preparation (Table 1.2).
A significant correlation between rivaroxaban plasma concentration and reaction time (R) have been reported for the specific anti-factor-Xa channel [AFXa] (strong to very strong correlation) (20, 21, 37), and for citrated kaolin channel (moderate to strong correlation) (35, 37).
The study of Kaaber et al. mentions that approximately 65% of the patients treated with rivaroxaban presented with RapidTEG™ activated clotting time within the normal reference, but that those with an activated clotting time above this level had a significantly increased risk of severe bleeding with high transfusion demands (33). Three studies did not find any significant effect of rivaroxaban on TEG 5000 parameters (31, 32, 42). One study compared the results descriptively only (46) (Table 2.2).
Rivaroxaban and ClotPro analyzer
Two studies reported results from the ClotPro analyzer (29, 38) (Supplementary Table 2.3). The commercially available Russel’s viper venom test (RVV-test) for the detection of factor Xa antagonists showed strong to very strong correlations between rivaroxaban plasma concentration and clotting time (CT) (29, 38) (Tables 1.3, 2.3).
Viscoelastic thresholds for rivaroxaban concentrations
Rivaroxaban plasma concentrations between 30 and 150 ng/ml can be detected by threshold values of the viscoelastic parameters CT and R (18–22, 24, 30, 38, 40) (Figure 5 and Tables 1.1–1.3). In particular, the RVV-test of ClotPro and the AFXa channel of TEG 6s show high sensitivity and specificity. It is important to mention that the study of Bliden et al. analyzed the results of rivaroxaban and apixaban in a pooled setting (22).
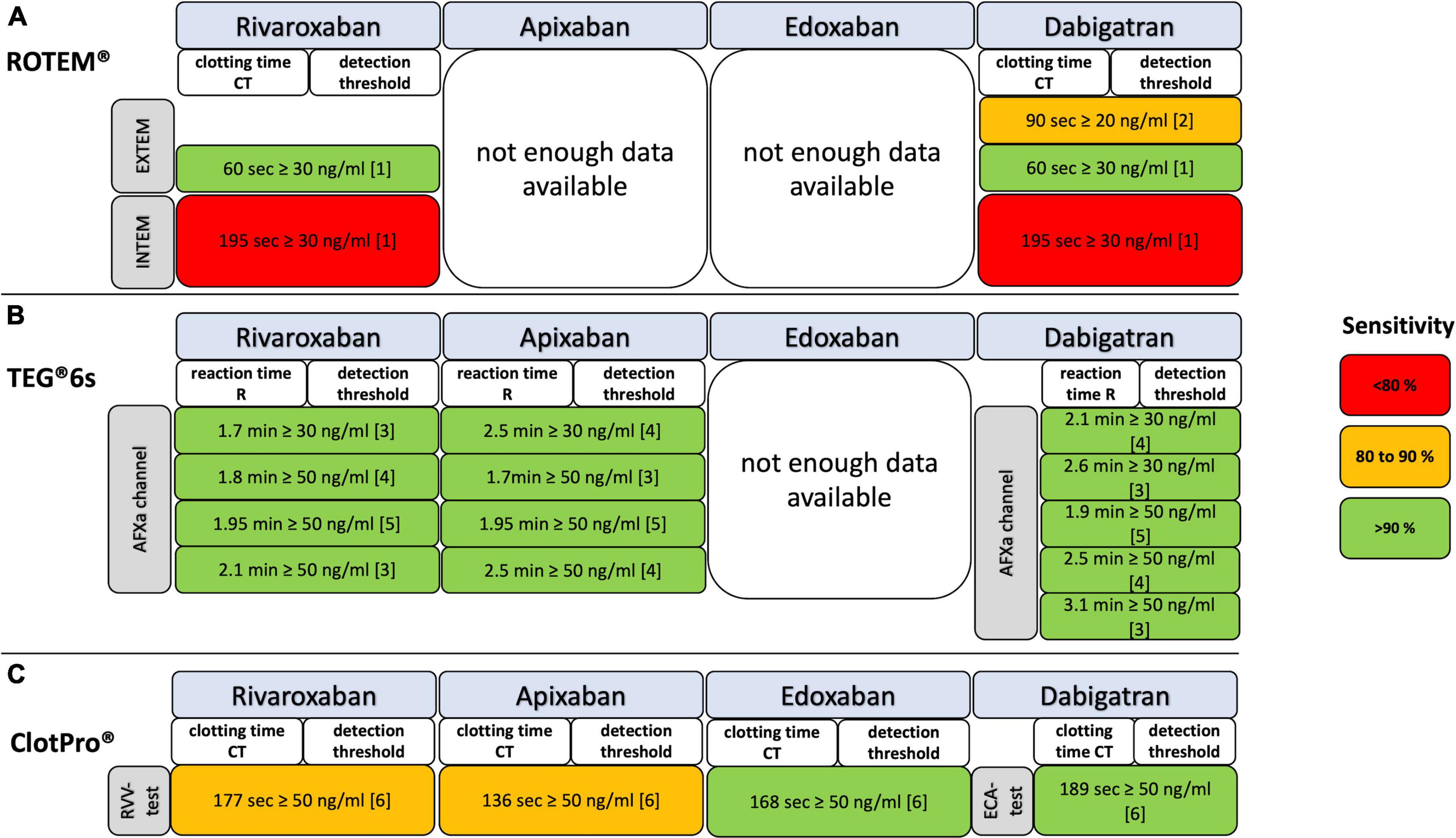
Figure 5. Detection of residual DOAC plasma concentrations with ROTEM, TEG, and ClotPro assays coded for test accuracy by sensitivity. (A) EXTEM, ROTEM assay extrinsic activated; INTEM, ROTEM assay intrinsic activated; NATEM, ROTEM assay natively activated; (B) DTI, direct thrombin inhibitor channel of TEG 6s; AFXa, anti-factor-Xa channel of TEG 6s; (C) ECA, ecarin-activated ClotPro test; RVV, Russell’s viper venom-activated ClotPro test. References: [1] Henskens et al. (30); [2] Taune et al. (69); [3] Artang et al. (21); [4] Artang et al. (20); [5] Bliden et al. (22); [6] Oberladstätter et al. (38).
Apixaban
Twenty-two studies were identified analyzing the effects if apixaban on viscoelastic testing (18, 20–22, 25–27, 29, 32, 33, 35, 38, 40, 45, 46, 48–54) (Supplementary Tables 2.1–2.3). The CT and R in relation to the apixaban plasma concentration are shown in Figure 4.
Apixaban and rotational thrombelastometry
We revealed nine studies assessing the effects of apixaban on ROTEM (18, 27, 40, 45, 48–52) (Supplementary Table 2.1). Out of these, three studies used modified or ad hoc designed assays (18, 40, 48), whereas the other studies focused on the EXTEM (27, 45, 49–52), INTEM (27, 45), NATEM (50) and FIBTEM (45) assays. Apixaban caused a statistically significant prolongation of CT in EXTEM (27, 49, 51), with a greater sensitivity than CT in INTEM (27, 45). In comparison to the other factor Xa inhibitors, apixaban had the lowest effect on CT, often requiring supra-therapeutic doses to achieve a significant CT prolongation (27, 45). Also, apixaban plasma levels beneath 50 ng/ml could not be detected by EXTEM CT changes (45, 51). The significance of a dose dependent effect of apixaban plasma concentrations on CT length was shown for EXTEM [strong correlation (45)], INTEM [strong correlation (45)], and NATEM [weak correlation (50)] assays (Table 2.1).
Our search identified four studies analyzing modified ROTEM assays (18, 40, 48, 50). Overall, the experimental changes to ROTEM resulted in CT prolongation, with some studies requiring lower concentrations than those done with commercially available channels (Table 1.1).
Apixaban and thrombelastography
Ten studies investigated the effects of apixaban on TEG with a focus on R value, using either the TEG 6s anti-factor Xa channel [AFXa] (20–22, 25), or TEG 5000 system (26, 32, 33, 35, 46, 53, 54) (Supplementary Table 2.2). A statistically significant, dose-dependent correlation of reaction time (R) and apixaban plasma concentration was shown with the anti-factor Xa channel [very strong correlation (20, 21)] and kaolin-TEG [moderate correlation (35)] (Tables 1.2, 2.2).
Two studies reported no statistically significant effect of apixaban plasma levels on reaction time (R) (32, 53).
Apixaban and ClotPro analyzer
Using the ClotPro analyzer, Oberladstätter et al. (38) found a statistically strong correlation between apixaban plasma concentrations and clotting time (CT), which, compared to the other anti-factor Xa inhibitors used in the study, showed weaker correlation (Tables 1.3, 2.3, and Supplementary Table 2.3).
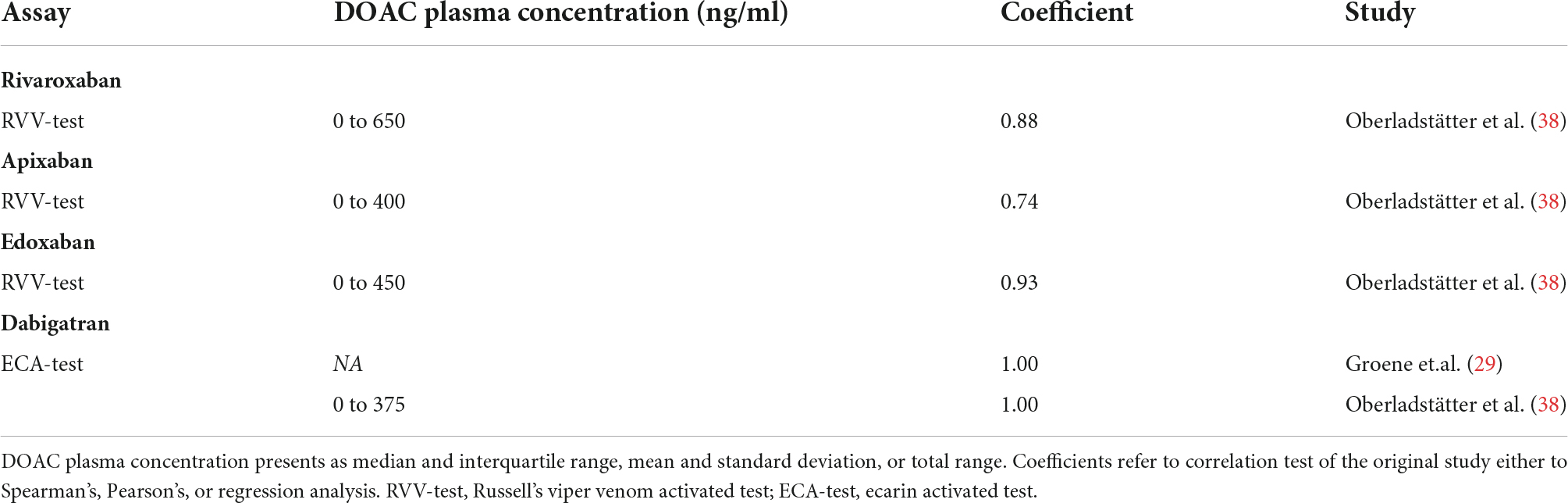
Table 2.3. Correlation of DOAC plasma concentration and ClotPro clotting time (CT) for different assays.
Viscoelastic thresholds for apixaban concentrations
There is no result with ROTEM EXTEM and INTEM for apixaban thresholds detection. Apixaban plasma concentrations of ≥30 and of ≥50 ng/ml can be detected by threshold values of the AFXa channel by TEG 6s and RVV-test by ClotPro (20–22, 38) (Figure 5 and Tables 1.1–1.3). No differences in R times between apixaban peak and through samples were found in another study (25).
Edoxaban
Six studies (25, 29, 38, 45, 46, 55) were identified analyzing the effects if edoxaban on viscoelastic testing (Supplementary Tables 2.1–2.3). The CT and R in relation to the edoxaban plasma concentration are shown in Figure 4.
Edoxaban and rotational thrombelastometry
By the time literature review was concluded there were only two studies assessing the effects of edoxaban on ROTEM showing significant prolongations of EXTEM clotting time (CT) even in therapeutic doses (45, 55) (Table 2.1).
Edoxaban and thrombelastography
Two studies described effects of edoxaban on the anti-factor-Xa channel [AFXa] of the TEG 6s and TEG 5000 system (Supplementary Table 2.2). The one study reported a pooled analysis of various DOACs together, not allowing for an individual result analysis (56). The other reported a change in parameters at doses several times higher than peak plasma levels (46) (Table 2.1).
Edoxaban and ClotPro analyzer
Alterations of ClotPro parameters by edoxaban were found in two studies (Supplementary Table 2.3). Main changes occurred to CT of the russel viper venom test, showing high sensitivity and specificity for a low threshold (38). Further, a statistically significant prolongation of the CT for this test was only seen in patients taking edoxaban compared to the other anti-factor Xa inhibitors rivaroxaban and apixaban (29) (Tables 1.3, 2.3).
Viscoelastic thresholds for edoxaban concentrations
Edoxaban plasma concentrations of 50 and 100 ng/ml can be detected by threshold values of clotting time (CT) (38) (Figure 5 and Table 1.2).
Viscoelastic analysis of direct factor II inhibitor
Dabigatran
29 studies described dabigatran action in whole blood with viscoelastic methods (20–22, 25–27, 29–31, 34, 35, 38, 45, 48, 57–71) (Supplementary Tables 2.1–2.3). As already mentioned for the factor Xa inhibitors, dabigatran showed either no significant influence or was not analyzed for other parameters except for the viscoelastic parameters clotting time (CT) and reaction time (R). The CT and R in relation to the dabigatran plasma concentration are shown in Figure 4.
Dabigatran and rotational thrombelastometry
Fourteen studies assessed dabigatran in whole blood with ROTEM (27, 30, 31, 45, 48, 58, 60, 61, 68–71) (Supplementary Table 2.1). Out of these, one study did not report the exact dabigatran concentration under which conditions the viscoelastic tests were performed (48). Most studies analyze the EXTEM (27, 30, 31, 34, 45, 58, 60, 64, 68–70) and INTEM (27, 30, 31, 34, 45, 58, 60, 64, 68, 70) original assays, with less data published on NATEM (61, 68) and FIBTEM (34, 45, 58, 70). Four studies reported results obtained from ad hoc designed or modified original assays (48, 60, 69, 70).
The majority of studies describe a correlation between plasma dabigatran concentrations with a linear increase of clotting time (CT), some highlighting a higher sensitivity of EXTEM over INTEM (27, 31, 34, 45, 58, 64, 68, 70, 72). In detail, a significant correlation between dabigatran plasma concentration and duration of clotting time (CT) varied from strong [EXTEM (34), INTEM (34, 70)] to very strong [EXTEM (45, 58, 64, 70), INTEM (45, 58, 60, 64) and FIBTEM (58, 70)] (Table 2.1).
Dabigatran and thrombelastography
Four studies investigated dabigatran action with the new cartridge-based direct thrombin inhibitor [DTI] channel of TEG 6s (20–22, 25), to our knowledge not yet commercially available at the time of manuscript preparation. Of these, two (22, 25) did not report the exact dabigatran concentration under which conditions the viscoelastic tests were performed.
The further studies investigated parameters with the TEG 5000 system (26, 31, 35, 57, 62, 63, 65–67) (Supplementary Table 2.2).
Dabigatran treated whole blood led to a significant prolongation of reaction time (R) when compared to baseline or control values in regard to the direct thrombin inhibitor channel (20), the citrated kaolin channel (26, 35, 67, 73), RapidTEG™ channel (26, 62), and calcium chloride (CaCl2) channel (63). One study did not find any effect of dabigatran on TEG 5000 parameters (57).
A significant correlation between dabigatran plasma concentration and reaction time (R) was shown for the direct thrombin inhibitor channel [very strong correlation (20, 21)], for the citrated kaolin channel [strong to very strong correlation (35, 65, 67)], and calcium chloride channel [moderate correlation (63)] (Table 2.2).
Dabigatran and ClotPro analyzer
The three studies performed with ClotPro showed moderate (59) to very high (29, 38) correlation between plasma dabigatran concentration and clotting time (CT) of pathway, specific for the detection of factor IIa antagonist (Supplementary Table 2.3).
Viscoelastic thresholds for dabigatran concentrations
Dabigatran plasma concentrations between 20 and 100 ng/ml can be detected by threshold values of the viscoelastic parameters CT and R (20–22, 30, 38, 69) (Figure 5; Tables 1.1–1.3). In particular, the ECA-test of ClotPro and the DTI channel of TEG 6s show consistent statistical sensitivity and specificity.
Impact of andexanet alfa and idarucizumab on viscoelastic testing
The specific antagonists andexanet alfa and idarucizumab have been developed for the reversal of direct oral anticoagulants. There is limited to no published information on viscoelastic coagulation testing for specific DOAC reversal (3) despite this method being of particular importance, as the treatment monitoring after administration of andexanet alfa should not be based on commercial anti-FXa activity assays (74, 75). In these assays, the FXa inhibitor dissociates from andexanet alfa resulting in the detection of falsely elevated anti-FXa activity levels.
We found two studies reporting viscoelastic testing after the specific reversal of DOAC (68, 76). No data is available for ROTEM on behalf of andexanet alfa. Takeshita et al. investigated the reversal of dabigatran by adding idarucizumab, which resulted in both INTEM and EXTEM clotting time reversal toward reference ranges (68). Oberladstätter et al. investigated the specific reversal of dabigatran with ClotPro ecarin clotting time (ECA-test CT) and apixaban, edoxaban, and rivaroxaban with ClotPro Russell’s viper venom test clotting time (RVV-test CT) (76). Idarucizumab substantially reduced ECA-CT, whereas andexanet alfa did not normalize the RVV-CT. Andexanet alfa spiking of non-anticoagulated blood prolonged RVV-CT, potentially as a consequence of a competitive antagonism with human factor Xa.
Discussion
This review shows the effect of the DOACs rivaroxaban, apixaban, edoxaban, and dabigatran on viscoelastic point-of-care tests. A total of 53 studies were included and qualitatively analyzed. Mainly, studies report ROTEM and TEG measurement methods, with rivaroxaban and dabigatran being the most studied.
Correlation of direct oral anticoagulant plasma concentration with viscoelastic tests
Direct oral anticoagulants (DOACs) show a clear influence on CT and R, resulting in being the main focus of studies. Other ROTEM and TEG parameters (e.g., MCF, A10, LI 60, or alpha angle) were either not further analyzed or showed no to minor changes in the reported studies. By using different activators, viscoelastic tests distinguish extrinsic, intrinsic or total pathways of coagulation in relation to the DOAC effect. The most specific results are produced by viscoelastic assays that reflect thrombin generation by measuring the physiological constitutional change of blood from the viscous to the clotted state. In principle, this reflects the length of CT as well as R. Accordingly, most significant concentration-dependent changes are described for ROTEM INTEM/EXTEM-CT and for TEG AFXa/DTI as well as CK channel R time. Overall, the results of the analyzed studies were trending toward DOACs showing a higher correlation of CT with drug concentration in the EXTEM channel over INTEM and FIBTEM. With TEG, the greatest affinity resulted with the AFXa or DTI channel, which are currently not yet available. Stronger correlations were demonstrated in assays with alternative not commercially available activators, but these are isolated examples and beyond the scope of this review. Not only is it important to consider the right cartridge and channel, but also the mechanism of action of the DOAC, distinguishing between dabigatran and factor-Xa inhibitors. In regards to the latter, the two most analyzed drugs, rivaroxaban and apixaban, show distinct differences in their affinity, even in identical conditions, potentially explaining the discrepancies in CT with apixaban (77). ROTEM tests were only poorly impacted by low levels of rivaroxaban, edoxaban or dabigatran, and apixaban had only a low effect even at high concentrations.
Detection of clinically relevant direct oral anticoagulant plasma concentrations with viscoelastic tests
Of particular interest are threshold values of clotting time CT or reaction time R at which a certain DOAC concentration must be assumed. It was shown that a cut-off value of 50 ng/ml does not exacerbate ongoing hemorrhage in bleeding patients (78, 79). For surgery with high bleeding risk, a preoperative DOAC concentration less than 30 ng/ml is proposed (78, 80, 81). In surgery with high expected blood loss, a calculated rivaroxaban concentration of greater than 100 ng/ml was associated with a significant increase of perioperative red blood cell loss (82). For thrombolysis in patients with acute ischemic stroke, plasma concentrations up to 100 ng/ml have been suggested to be acceptable (83). According to current guidelines, administration of reversal agents in bleeding patients on DOACs should be considered if plasma concentrations exceed 50 ng/ml (80). Most of the research regarding perioperative bleeding thresholds focuses on rivaroxaban, as this is the most prescribed DOAC (79, 81). We are not aware of any study investigating the interchangeability of above mentioned perioperative bleeding thresholds between different DOACS.
Most data are available for rivaroxaban and dabigatran. We revealed a good sensitivity of viscoelastic parameters in patients using DOAC and might therefore be a good candidate for emergency testing. The added advantage is that the results are readily available and there is uniform performance. But it must be assumed that viscoelastic methods are not sensitive enough to determine specific DOAC concentrations. Results within the normal reference range do not reliably exclude relevant residual DOAC plasma levels and limit their clinical implications. Further, traditional viscoelastic coagulation monitoring assays were not designed to measure the effects of DOACs. Of interest, the costs of DOAC specific laboratory measurements, such as anti-Xa-activity or liquid-chromatography mass-spectrometry (a more accurate method compared to HP-LC) are $40USD and $130USD, with a turnaround time of approximately 30–60 min and 2-4 h, respectively. Moreover, liquid-chromatography mass-spectrometry measurements may not be available 24/7. Viscoelastic tests cost on average about $70USD per analysis and provide first results within minutes. However, there may be price differences depending on the manufacturer and country-specific health system.
Monitoring the specific direct oral anticoagulant reversal
The use of commercially available anti-FXa assays to measure rivaroxaban or apixaban concentrations in patients after reversal with andexanet alfa has limitations. One of the limitations is the large sample dilution in the assay set-up, which causes dissociation of the inhibitor from the andexanet alfa-inhibitor complex, resulting in an erroneous elevation of the anti-FXa activity (5). For rivaroxaban, the residual drug concentration 4 h after treatment with andexanet alfa was approximately 42% lower than the pretreatment concentration (84). A concentration that can still affect hemostasis. Thus, viscoelastic testing may play an important role in monitoring after andexanet alfa reversal (85).
For dabigatran reversal, there appears to be a rebound or dissociation effect after 12 to 24 h (5). Measurements of dabigatran may predict the need for secondary dosing of this reversal agent. In a retrospective study, it has been shown that no plasma dabigatran rebound was observed after reversal in patients with dabigatran plasma level <264 ng/mL at baseline (86). Further, in a case of ongoing bleeding by chronic accumulation of dabigatran showed impressively ongoing redistribution of dabigatran necessitates repetitive application of idarucizumab to neutralize dabigatran (87). Accordingly, repeated and timely coagulation monitoring is required.
Limitation
The studies are heterogeneous, and their replication of results was not constant. For apixaban in particular, there are heterogeneous data for the effect on CT EXTEM in ROTEM. This may be attributed to methodological differences in the work of Escolar et al. and Pujadas-Mestres et al. (49, 51). Most studies are based on a small study population or sample collection. The analyses reflect different populations including healthy volunteers with spiked samples and in vitro analyses. Furthermore, there is a lack of international reference values among the different tests. On the other hand, this work includes a large number of studies. Direct conclusions for the treatment of patients under DOAC can be made for clinical use from this review. We omitted betrixaban from our review as the drug was discontinued by the manufacturer in April 2020 for independent business reasons and never received approval from the European Medicines Agency.
Conclusion
Viscoelastic test assays can provide fast and essential point-of-care information regarding residual DOAC activity, especially DOAC specific assays. Even with strong correlation between the DOAC plasma concentration and viscoelastic parameter clotting time (CT) or reaction time (R), the results could be within the normal reference range. The quantification of residual DOAC plasma concentration with DOAC unspecific viscoelastic assays is not sensitive enough, compared with recommended anti-Xa activity laboratory measurements.
Data availability statement
The original contributions presented in the study are included in the article/Supplementary material, further inquiries can be directed to the corresponding author.
Author contributions
SDS, DRS, and AK contributed to conception and design of the study. SDS and CC organized the database. SDS wrote the first draft and processed the manuscript. CC wrote sections of the manuscript. All authors contributed to manuscript revision, read, and approved the submitted version.
Acknowledgments
DRS acknowledges grant support from the Swiss National Science Foundation, the Swiss Society of Anesthesiology and Perioperative Medicine (SSAPM), and the Swiss Foundation for Anesthesia Research via his department.
Conflict of interest
AK received honoraria for lecturing from Bayer AG Switzerland. DRS’s academic department receives grant support from Vifor SA, and Vifor (International) AG. He is co-chair of the ABC-Trauma Faculty, sponsored by unrestricted educational grants from Novo Nordisk Health Care AG, CSL Behring GmbH, LFB Biomédicaments, and Octapharma AG. He has received honoraria/travel support for consulting or lecturing from: Alexion Pharmaceuticals Inc., AstraZeneca AG, Bayer AG, B. Braun Melsungen AG, CSL Behring GmbH, Celgene International II Sàrl, Daiichi Sankyo AG, Haemonetics, Instrumentation Laboratory (Werfen), LFB Biomédicaments, Merck Sharp & Dohme, Novo Nordisk Health Care AG, PAION Deutschland GmbH, Pharmacosmos A/S, Pfizer AG, Pierre Fabre Pharma, Portola Schweiz GmbH, Roche Diagnostics International Ltd, Sarstedt AG & Co., Shire Switzerland GmbH, Takeda, Tem International GmbH, Vifor Pharma, Vifor (International) AG, and Zuellig Pharma Holdings.
The remaining authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fcvm.2022.991675/full#supplementary-material
References
1. Eikelboom JW, Connolly SJ, Bosch J, Dagenais GR, Hart RG, Shestakovska O, et al. Rivaroxaban with or without aspirin in stable cardiovascular disease. New England J Med. (2017) 377:1319–30.
2. Eikelboom JW, Quinlan DJ, Hirsh J, Connolly SJ, Weitz JI. Laboratory monitoring of non-vitamin k antagonist oral anticoagulant use in patients with atrial fibrillation: a review. JAMA Cardiol. (2017) 2:566–74.
3. Gosselin RC, Adcock DM, Douxfils J. An update on laboratory assessment for direct oral anticoagulants (DOACs). Int J Laborat Hematol. (2019) 41:33–9.
4. Ziakas PD, Mylonakis E. Web search popularity, publicity, and utilization of direct oral anticoagulants in the United States, 2008-2018: a STROBE-compliant study. Medicine. (2020) 99:e20005. doi: 10.1097/MD.0000000000020005
5. Douxfils J, Adcock DM, Bates SM, Favaloro EJ, Gouin-Thibault I, Guillermo C, et al. 2021 update of the international council for standardization in haematology recommendations for laboratory measurement of direct oral anticoagulants. Throm Haemost. (2021) 121:1008–20. doi: 10.1055/a-1450-8178
6. Weitz JI, Eikelboom JW. Urgent need to measure effects of direct oral anticoagulants. Circulation. (2016) 134:186–8.
7. Samuelson BT, Cuker A, Siegal DM, Crowther M, Garcia DA. Laboratory assessment of the anticoagulant activity of direct oral anticoagulants: a systematic review. Chest. (2017) 151:127–38.
8. Gosselin RC, Adcock D, Dorgalaleh A, Favaloro EJ, Lippi G, Pego JM, et al. International council for standardization in haematology recommendations for hemostasis critical values, tests, and reporting. Sem Throm Hemost. (2020) 46:398–409. doi: 10.1055/s-0039-1697677
9. Spyropoulos AC, Al-Badri A, Sherwood MW, Douketis JD. To measure or not to measure direct oral anticoagulants before surgery or invasive procedures: comment. J Throm Haemost JTH. (2016) 14:2556–9.
10. Douxfils J, Ageno W, Samama CM, Lessire S, Ten Cate H, Verhamme P, et al. Laboratory testing in patients treated with direct oral anticoagulants: a practical guide for clinicians. J Throm Haemost JTH. (2018) 16:209–19.
11. Stein P, Kaserer A, Sprengel K, Wanner GA, Seifert B, Theusinger OM, et al. Change of transfusion and treatment paradigm in major trauma patients. Anaesthesia. (2017) 72:1317–26.
12. Spahn DR, Kaserer A, Studt JD. Coagulation management after trauma in the presence of direct oral anticoagulants. Anesthesiology. (2021) 135:570–2.
13. Sahli SD, Rössler J, Tscholl DW, Studt JD, Spahn DR, Kaserer A. Point-of-care diagnostics in coagulation management. Sensors. (2020) 20:15.
14. Wells GA, O’Connell D, Peterson J, Welch V, Losos M, Tugwell P. The newcastle-ottawa scale (NOS) for assessing the quality of nonrandomised studies in meta-analyses. (2003). Available online at: http://www.ohri.ca/programs/clinical_epidemiology/oxford.asp (accessed March 31, 2022).
16. Einfache lineare Regression. (1992). Available online at: https://www.methodenberatung.uzh.ch/de/datenanalyse_spss/zusammenhaenge/ereg.html#3.7._Berechnung_der_Effektst%C3%A4rke (accessed December 6, 2021).
17. Nakagawa S, Cuthill IC. Effect size, confidence interval and statistical significance: a practical guide for biologists. Biol Rev Camb Philos Soc. (2007) 82:591–605. doi: 10.1111/j.1469-185X.2007.00027.x
18. Adelmann D, Wiegele M, Wohlgemuth RK, Koch S, Frantal S, Quehenberger P, et al. Measuring the activity of apixaban and rivaroxaban with rotational thrombelastometry. Throm Res. (2014) 134:918–23. doi: 10.1016/j.thromres.2014.08.006
19. Aranda VF, Derogis PBM, Sanches LR, Mangueira CLP, Katz M, Faulhaber ACL, et al. Diagnostic accuracy of thromboelastometry and its correlation with the HPLC-MS/MS quantification test. Braz J Med Biol Res Rev Brasil De Pesquisas Med Biol. (2019) 52:e8006. doi: 10.1590/1414-431X20198006
20. Artang R, Anderson M, Nielsen JD. Fully automated thromboelastograph TEG 6s to measure anticoagulant effects of direct oral anticoagulants in healthy male volunteers. Res Pract Throm Haemost. (2019) 3:391–6. doi: 10.1002/rth2.12206
21. Artang R, Dias JD, Walsh M, Bliden K, Nielsen JD, Anderson M, et al. Measurement of anticoagulation in patients on dabigatran, rivaroxaban, and apixaban therapy by novel automated thrombelastography. Open. (2021) 5:e570–6. doi: 10.1055/a-1692-1415
22. Bliden KP, Chaudhary R, Mohammed N, Muresan AA, Lopez-Espina CG, Cohen E, et al. Determination of non-vitamin K oral anticoagulant (NOAC) effects using a new-generation thrombelastography TEG 6s system. J Thromb Thromb. (2017) 43:437–45. doi: 10.1007/s11239-017-1477-1
23. Casutt M, Konrad C, Schuepfer G. Effect of rivaroxaban on blood coagulation using the viscoelastic coagulation test ROTEM™. Der Anaesthesist. (2012) 61:948–53. doi: 10.1007/s00101-012-2091-4
24. Chojnowski K, Górski T, Robak M, Treliński J. Effects of rivaroxaban therapy on ROTEM coagulation parameters in patients with venous thromboembolism. Adv Clin Exp Med. (2015) 24:995–1000. doi: 10.17219/acem/42147
25. Dias JD, Lopez-Espina CG, Ippolito J, Hsiao LH, Zaman F, Muresan AA, et al. Rapid point-of-care detection and classification of direct-acting oral anticoagulants with the TEG 6s: implications for trauma and acute care surgery. J Trauma Acute Care Surg. (2019) 87:364–70. doi: 10.1097/TA.0000000000002357
26. Dias JD, Norem K, Doorneweerd DD, Thurer RL, Popovsky MA, Omert LA. Use of thromboelastography (TEG) for detection of new oral anticoagulants. Arch Pathol Laborat Med. (2015) 139:665–73.
27. Eller T, Busse J, Dittrich M, Flieder T, Alban S, Knabbe C, et al. Dabigatran, rivaroxaban, apixaban, argatroban and fondaparinux and their effects on coagulation POC and platelet function tests. Clin Chem Laborat Med. (2014) 52:835–44. doi: 10.1515/cclm-2013-0936
28. Fontana P, Alberio L, Angelillo-Scherrer A, Asmis LM, Korte W, Mendez A, et al. Impact of rivaroxaban on point-of-care assays. Throm Res. (2017) 153:65–70.
29. Groene P, Wagner D, Kammerer T, Kellert L, Giebl A, Massberg S, et al. Viscoelastometry for detecting oral anticoagulants. Throm J. (2021) 19:18. doi: 10.1186/s12959-021-00267-w
30. Henskens YMC, Gulpen AJW, van Oerle R, Wetzels R, Verhezen P, Spronk H, et al. Detecting clinically relevant rivaroxaban or dabigatran levels by routine coagulation tests or thromboelastography in a cohort of patients with atrial fibrillation. Throm J. (2018) 16:3.
31. Herrmann R, Thom J, Wood A, Phillips M, Muhammad S, Baker R. Thrombin generation using the calibrated automated thrombinoscope to assess reversibility of dabigatran and rivaroxaban. Throm Haemost. (2014) 111:989–95. doi: 10.1160/TH13-07-0607
32. Jenrette J, Schwarz K, Trujillo T, Ray L. Evaluation of direct oral anticoagulant use on thromboelastography in an emergency department population. Am J Emerg Med. (2022) 52:191–5. doi: 10.1016/j.ajem.2021.12.011
33. Kaaber AB, Jans Ø, Dziegiel MH, Stensballe J, Johansson PI. Managing patients on direct factor Xa inhibitors with rapid thrombelastography. Scand J Clin Laborat Investigat. (2021) 81:661–9.
34. Klages M, Raimann FJ, Philipp AL, Lindhoff-Last E, Zacharowski K, Mutlak H. Direct oral anticoagulants in point-of-care monitoring: an ex-vivo study. Minerva Anestesiol. (2021) 87:514–22.
35. Kopytek M, Zabczyk M, Natorska J, Malinowski KP, Undas A. Effects of direct oral anticoagulants on thromboelastographic parameters and fibrin clot properties in patients with venous thromboembolism. J Physiol Pharmacol offi J Polish Physiol Soc. (2020) 71:1. doi: 10.26402/jpp.2020.1.03
36. Körber MK, Langer E, Ziemer S, Perzborn E, Gericke C, Von Heymann C. Measurement and reversal of prophylactic and therapeutic peak levels of rivaroxaban: an in vitro study. Clin Appl Throm Hemost. (2014) 20:735–40. doi: 10.1177/1076029613494468
37. Myers SP, Dyer MR, Hassoune A, Brown JB, Sperry JL, Meyer MP, et al. Correlation of thromboelastography with apparent rivaroxaban concentration: has point-of-care testing improved? Anesthesiology. (2020) 132:280–90. doi: 10.1097/ALN.0000000000003061
38. Oberladstätter D, Voelckel W, Schlimp C, Zipperle J, Ziegler B, Grottke O, et al. A prospective observational study of the rapid detection of clinically-relevant plasma direct oral anticoagulant levels following acute traumatic injury. Anaesthesia. (2020) 76:373–80. doi: 10.1111/anae.15254
39. Oswald E, Velik-Salchner C, Innerhofer P, Tauber H, Auckenthaler T, Ulmer H, et al. Results of rotational thromboelastometry, coagulation activation markers and thrombin generation assays in orthopedic patients during thromboprophylaxis with rivaroxaban and enoxaparin: a prospective cohort study. Blood Coagulat Fibrinol. (2015) 26:136–44. doi: 10.1097/MBC.0000000000000203
40. Pailleret C, Jourdi G, Siguret V, Gouin-Thibault I, Gandrille S, Stepanian A, et al. Modified ROTEM for the detection of rivaroxaban and apixaban anticoagulant activity in whole blood: a diagnostic test study. Eur J Anaesthesiol. (2019) 36:449–56. doi: 10.1097/EJA.0000000000000903
41. Perzborn E, Heitmeier S, Laux V, Buchmüller A. Reversal of rivaroxaban-induced anticoagulation with prothrombin complex concentrate, activated prothrombin complex concentrate and recombinant activated factor VII in vitro. Thromb Res. (2014) 133:671–81.
42. Rathbun S, Tafur A, Grant R, Esmon N, Mauer K, Marlar RA. Comparison of methods to determine rivaroxaban anti-factor Xa activity. Throm Res. (2015) 135:394–7.
43. Samama MM, Martinoli JL, LeFlem L, Guinet C, Plu-Bureau G, Depasse F, et al. Assessment of laboratory assays to measure rivaroxaban - an oral, direct factor Xa inhibitor. Throm Haemost. (2010) 103:815–25.
44. Schenk B, Würtinger P, Streif W, Sturm W, Fries D, Bachler M. Ex vivo reversal of effects of rivaroxaban evaluated using thromboelastometry and thrombin generation assay. Br J Anaesthe. (2016) 117:583–91.
45. Seyve L, Richarme C, Polack B, Marlu R. Impact of four direct oral anticoagulants on rotational thromboelastometry (ROTEM). Int J Laborat Hematol. (2018) 40:84–93. doi: 10.1111/ijlh.12744
46. Siddiqui F, Hoppensteadt D, Jeske W, Iqbal O, Tafur A, Fareed J. Factor xa inhibitory profile of apixaban, betrixaban, edoxaban, and rivaroxaban does not fully reflect their biologic spectrum. Clin Appl Throm Hemost Offi J Int Acad Clin Appl Throm Hemost. (2019) 25:1076029619847524. doi: 10.1177/1076029619847524
47. Tsantes AE, Kyriakou E, Ikonomidis I, Katogiannis K, Papadakis I, Douramani P, et al. Comparative assessment of the anticoagulant activity of rivaroxaban and dabigatran in patients with nonvalvular atrial fibrillation: a noninterventional study. Medicine. (2016) 95:e3037. doi: 10.1097/MD.0000000000003037
48. Vedovati MC, Mosconi MG, Isidori F, Agnelli G, Becattini C. Global thromboelastometry in patients receiving direct oral anticoagulants: the RO-DOA study. J Throm Thrombol. (2020) 49:251–8. doi: 10.1007/s11239-019-01956-0
49. Escolar G, Fernandez-Gallego V, Arellano-Rodrigo E, Roquer J, Reverter JC, Sanz VV, et al. Reversal of apixaban induced alterations in hemostasis by different coagulation factor concentrates: significance of studies In vitro with circulating human blood. PLoS One. (2013) 8:11. doi: 10.1371/journal.pone.0078696
50. Kyriakou E, Katogiannis K, Ikonomidis I, Giallouros G, Nikolopoulos GK, Rapti E, et al. Laboratory assessment of the anticoagulant activity of apixaban in patients with nonvalvular atrial fibrillation. Clin Appl Throm Hemost. (2018) 24:194S–201S.
51. Pujadas-Mestres L, Lopez-Vilchez I, Arellano-Rodrigo E, Reverter JC, Lopez-Farre A, Diaz-Ricart M, et al. Differential inhibitory action of apixaban on platelet and fibrin components of forming thrombi: studies with circulating blood and in a platelet-based model of thrombin generation. PLoS One. (2017) 12:e0171486. doi: 10.1371/journal.pone.0171486
52. Schmidt K, Krüger K, Langer E, Schmutzler M, Johnen E, Wernecke KD, et al. Reversal of apixaban induced alterations in haemostasis by different coagulation factor concentrates in patients after hip or knee replacement surgery. Blood Trans Trasfusione del Sangue. (2019) 17:157–62.
53. Spinthakis N, Gue Y, Farag M, Srinivasan M, Wellsted D, Arachchillage DRJ, et al. Apixaban enhances endogenous fibrinolysis in patients with atrial fibrillation. Eur Pacing Arrhyth Card Electrophysiol J Work Groups Card Pacing Arrhyth Card Cell Electrophysiol Eur Soc Cardiol. (2019) 21:1297–306.
54. Voukalis C, Lip GYH, Shantsila E. Effects of antithrombotic drugs on the prothrombotic state in patients with atrial fibrillation: the west birmingham atrial fibrillation project. Throm Res. (2021) 200:149–55. doi: 10.1016/j.thromres.2021.02.005
55. Havrdová M, Saari TI, Jalonen J, Peltoniemi M, Kurkela M, Vahlberg T, et al. Relationship of edoxaban plasma concentration and blood coagulation in healthy volunteers using standard laboratory tests and viscoelastic analysis. J Clin Pharmacol. (2020) 61:522–30. doi: 10.1002/jcph.1758
56. Dias JD, Lopez-Espina C, Ippolito J, Hsiao H, Zaman F, Mathew B, et al. Novel thromboelastography point-of-care test detects all commercially available DOACs at therapeutic concentrations and classifies them as direct thrombin or factor xa inhibitors with high consistency. Eur Heart J. (2018) 39:19.
57. Aho A, Byrne K. The effect of dabigatran on the kaolin-activated whole blood thromboelastogram. Anaesth Int Care. (2016) 44:729–33. doi: 10.1177/0310057X1604400607
58. Comuth WJ, Henriksen L, van de Kerkhof D, Husted SE, Kristensen SD, de Maat MPM, et al. Comprehensive characteristics of the anticoagulant activity of dabigatran in relation to its plasma concentration. Throm Res. (2018) 164:32–9. doi: 10.1016/j.thromres.2018.02.141
59. Fong AYY, Tiong LL, Tan SSN, Geruka D, Apil GG, Choo CW, et al. Effect of dabigatran on clotting time in the clotpro ecarin clotting assay: a prospective, single-arm, open-label study. Clin Appl Thromb Hemost Offi J Int Acad Clin Appl Throm Hemost. (2020) 26:1076029620972473. doi: 10.1177/1076029620972473
60. Körber MK, Langer E, Köhr M, Wernecke KD, Korte W, Von Heymann C. In vitro and ex vivo measurement of prophylactic dabigatran concentrations with a new ecarin-based thromboelastometry test. Trans Med Hemother. (2017) 44:100–5. doi: 10.1159/000470622
61. Kyriakou E, Ikonomidis I, Stylos D, Bonovas S, Papadakis I, Nikolopoulos GK, et al. Laboratory assessment of the anticoagulant activity of dabigatran. Clin Appl Throm Hemost. (2015) 21:434–45.
62. Nadtochiy SM, Baldzizhar A, Stefanos T, Feng C, O’Leary KE, Jones-Smith KL, et al. High-dose dabigatran is an effective anticoagulant for simulated cardiopulmonary bypass using human blood. Anesthe Anal. (2020) 132:566–74. doi: 10.1213/ANE.0000000000005089
63. Pipilis A, Makrygiannis S, Anagnostou G, Kaliampakos S, Tsakonas G, Sourlas N, et al. Dabigatran plasma levels, aPTT and thromboelastography in patients with AF: implications for allowing early non-elective surgical procedures. J Throm Thrombol. (2017) 44:9–13. doi: 10.1007/s11239-017-1503-3
64. Sokol J, Nehaj F, Ivankova J, Mokan M, Zolkova J, Lisa L, et al. Impact of dabigatran treatment on rotation thromboelastometry. Clin Appl Throm Hemost Offi J Int Acad Clin Appl Throm Hemost. (2021) 27:1076029620983902.
65. Solbeck S, Jensen AS, Maschmann C, Stensballe J, Ostrowski SR, Johansson PI. The anticoagulant effect of therapeutic levels of dabigatran in atrial fibrillation evaluated by thrombelastography (TEG(®)), hemoclot thrombin inhibitor (HTI) assay and ecarin clotting time (ECT). Scand J Clin Laborat Investigat. (2018) 78:25–30. doi: 10.1080/00365513.2017.1408138
66. Solbeck S, Meyer MA, Johansson PI, Meyer AS, Cotton BA, Stensballe J, et al. Monitoring of dabigatran anticoagulation and its reversal in vitro by thrombelastography. Int J Cardiol. (2014) 176:794–9.
67. Solbeck S, Ostrowski SR, Stensballe J, Johansson PI. Thrombelastography detects dabigatran at therapeutic concentrations in vitro to the same extent as gold-standard tests. Int J Cardiol. (2016) 208:14–8. doi: 10.1016/j.ijcard.2016.01.148
68. Takeshita S, Tanaka KA, Sawa T, Sanda M, Mizobe T, Ogawa S. Whole blood point-of-care testing for incomplete reversal with idarucizumab in supratherapeutic dabigatran. Anesth Analg. (2020) 130:535–41. doi: 10.1213/ANE.0000000000004419
69. Taune V, Skeppholm M, Ågren A, Gryfelt G, Malmström RE, Wikman A, et al. Rapid determination of anticoagulating effects of dabigatran in whole blood with rotational thromboelastometry and a thrombin-based trigger. J Throm Haemost JTH. (2018) 16:2462–70. doi: 10.1111/jth.14308
70. Taune V, Wallén H, Ågren A, Gryfelt G, Sjövik C, Wintler AM, et al. Whole blood coagulation assays ROTEM and T-TAS to monitor dabigatran treatment. Throm Res. (2017) 153:76–82. doi: 10.1016/j.thromres.2017.03.018
71. Tsantes AE, Kyriakou E, Bonovas S, Chondrogianni M, Zompola C, Liantinioti C, et al. Impact of dabigatran on platelet function and fibrinolysis. J Neurol Sci. (2015) 357:204–8. doi: 10.1016/j.jns.2015.07.031
72. Henskens Y, Van Oerle R, Wetzels R, Spronk H, Schalla S, Crijns H, et al. Detecting relevant rivaroxaban or dabigatran levels in clinical care by routine coagulation tests or thromboelastography in patients with atrial fibrillation. J Throm Haemost. (2015) 13:902–3.
73. Solbeck S, Nilsson CU, Engström M, Ostrowski SR, Johansson PI. Dabigatran and its reversal with recombinant factor VIIa and prothrombin complex concentrate: a sonoclot in vitro study. Scand J Clin Laborat Investigat. (2014) 74:591–8. doi: 10.3109/00365513.2014.921930
74. Portola. Ondexxya (andexanet alfa): commercial anti-FXa activity assays are unsuitable for measuring anti-FXa activity following administration of andexanet alfa. Amsterdam: European Medicines Agency (2020).
75. Langer A, Connors JM. Assessing and reversing the effect of direct oral anticoagulants on coagulation. Anesthesiology. (2020) 133:223–32.
76. Oberladstätter D, Schlimp CJ, Zipperle J, Osuchowski MF, Voelckel W, Grottke O, et al. Impact of idarucizumab and andexanet alfa on DOAC plasma concentration and ClotPro(®) clotting time: an ex vivo spiking study in a cohort of trauma patients. J Clin Med. (2021) 10:16. doi: 10.3390/jcm10163476
77. Jourdi G, Siguret V, Martin AC, Golmard JL, Godier A, Samama CM, et al. Association rate constants rationalise the pharmacodynamics of apixaban and rivaroxaban. Throm Haemost. (2015) 114:78–86. doi: 10.1160/TH14-10-0877
78. Pernod G, Albaladejo P, Godier A, Samama CM, Susen S, Gruel Y, et al. Management of major bleeding complications and emergency surgery in patients on long-term treatment with direct oral anticoagulants, thrombin or factor-xa inhibitors: proposals of the working group on perioperative haemostasis (GIHP) - march 2013. Arch Cardiovasc Dis. (2013) 106:382–93.
79. Douketis JD, Spyropoulos AC, Duncan J, Carrier M, Le Gal G, Tafur AJ, et al. Perioperative management of patients with atrial fibrillation receiving a direct oral anticoagulant. JAMA Int Med. (2019) 179:1469–78.
80. Levy JH, Ageno W, Chan NC, Crowther M, Verhamme P, Weitz JI. When and how to use antidotes for the reversal of direct oral anticoagulants: guidance from the SSC of the ISTH. J Throm Haemost JTH. (2016) 14:623–7. doi: 10.1111/jth.13227
81. Godier A, Dincq AS, Martin AC, Radu A, Leblanc I, Antona M, et al. Predictors of pre-procedural concentrations of direct oral anticoagulants: a prospective multicentre study. Eur Heart J. (2017) 38:2431–9. doi: 10.1093/eurheartj/ehx403
82. Kaserer A, Kiavialaitis GE, Braun J, Schedler A, Stein P, Rössler J, et al. Impact of rivaroxaban plasma concentration on perioperative red blood cell loss. Transfusion. (2020) 60:197–205. doi: 10.1111/trf.15560
83. Seiffge DJ, Traenka C, Polymeris AA, Thilemann S, Wagner B, Hert L, et al. Intravenous thrombolysis in patients with stroke taking rivaroxaban using drug specific plasma levels: experience with a standard operation procedure in clinical practice. J Stroke. (2017) 19:347–55. doi: 10.5853/jos.2017.00395
84. Connolly SJ, Crowther M, Eikelboom JW, Gibson CM, Curnutte JT, Lawrence JH, et al. Full study report of andexanet alfa for bleeding associated with factor xa inhibitors. New England J Med. (2019) 380:1326–35. doi: 10.1056/NEJMoa1814051
85. Pavoni V, Gianesello L, Conti D, Ballo P, Dattolo P, Prisco D, et al. “In less than no time”: feasibility of rotational thromboelastometry to detect anticoagulant drugs activity and to guide reversal therapy. J Clin Med. (2022) 11:1407. doi: 10.3390/jcm11051407
86. Gendron N, Chocron R, Billoir P, Brunier J, Camoin-Jau L, Tuffigo M, et al. Dabigatran level before reversal can predict hemostatic effectiveness of idarucizumab in a real-world setting. Front Med. (2020) 7:599626. doi: 10.3389/fmed.2020.599626
87. Hegemann I, Ganter C, Widmer CC, Becker M, Müller D, Spahn DR. Ongoing redistribution of dabigatran necessitates repetitive application of idarucizumab. Br J Anaesth. (2018) 121:505–8. doi: 10.1016/j.bja.2018.04.025
88. Lang T, Bauters A, Braun SL, Pötzsch B, von Pape KW, Kolde HJ, et al. Multi-centre investigation on reference ranges for ROTEM thromboelastometry. Blood Coagulat Fibrinol Int J Haemost Throm. (2005) 16:301–10.
89. Stein P, Kaserer A, Spahn GH, Spahn DR. Point-of-care coagulation monitoring in trauma patients. Sem Throm Hemost. (2017) 43:367–74.
Keywords: DOAC, point-of-care, ROTEM, TEG, ClotPro, FXa inhibitor, FII inhibitor
Citation: Sahli SD, Castellucci C, Roche TR, Rössler J, Spahn DR and Kaserer A (2022) The impact of direct oral anticoagulants on viscoelastic testing – A systematic review. Front. Cardiovasc. Med. 9:991675. doi: 10.3389/fcvm.2022.991675
Received: 26 July 2022; Accepted: 03 October 2022;
Published: 07 November 2022.
Edited by:
Jianyong Ma, University of Cincinnati, United StatesReviewed by:
Dimitrios Tsakiris, University of Basel, SwitzerlandNicolas Gendron, Hôpital Européen Georges Pompidou, France
Copyright © 2022 Sahli, Castellucci, Roche, Rössler, Spahn and Kaserer. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Alexander Kaserer, alexander.kaserer@usz.ch
†These authors share first authorship
 Sebastian D. Sahli
Sebastian D. Sahli Clara Castellucci
Clara Castellucci Tadzio R. Roche1
Tadzio R. Roche1  Julian Rössler
Julian Rössler