Published online Feb 28, 2014. doi: 10.3748/wjg.v20.i8.2107
Revised: December 15, 2013
Accepted: January 3, 2014
Published online: February 28, 2014
AIM: To investigate the utility of phosphorus-31 (31P) magnetic resonance spectroscopy (MRS) as a noninvasive test for assessment of response to interferon and ribavirin treatment in patients with different severities of hepatitis C virus infection.
METHODS: Sixty chronic hepatitis C patients undergoing antiviral therapy with interferon and ribavirin underwent 31P MRS at 3.0T before treatment, 6 mo after the start of treatment, and 1 year after the start of treatment.
RESULTS: The phosphomonoester (PME)/phosphodiester (PDE) ratio at 6 mo after the start of antiviral therapy in the Child-Pugh B and C groups were significantly higher than those before therapy, but this was not seen in the Child-Pugh A group. In the antiviral therapy group, the PME/PDE ratios had decreased on follow-up MR spectroscopy. However, in the virological nonresponder group, the PME/PDE ratios on follow-up imaging were similar to the baseline values.
CONCLUSION: 31P MRS can be used to provide biochemical information on hepatic metabolic processes. This study indicates that the PME/PDE ratio can be used as an indicator of response to antiviral treatment in chronic hepatitis C patients.
Core tip: This study assessed the value of 3.0T 31P magnetic resonance spectroscopy, a noninvasive technique, in testing response to antiviral therapy for chronic hepatitis C. The technique can provide biochemical information on hepatic metabolic processes. The phosphomonoester/phosphodiester ratio can be used as an indicator of response to antiviral treatment in chronic hepatitis C patients.
- Citation: Zhang CY, Zhang Q, Zhang HM, Yang HS. 3.0T 31P MR spectroscopy in assessment of response to antiviral therapy for chronic hepatitis C. World J Gastroenterol 2014; 20(8): 2107-2112
- URL: https://www.wjgnet.com/1007-9327/full/v20/i8/2107.htm
- DOI: https://dx.doi.org/10.3748/wjg.v20.i8.2107
Hepatitis C virus (HCV) is one of the leading causes of liver disease worldwide. It is estimated that approximately 3% of the global population is infected with HCV. Many of the cases develop into chronic liver disease, cirrhosis, or even hepatocellular carcinoma[1]. Liver biopsy remains the gold standard for providing the stage (extent of fibrosis) and grade (degree of NI activity) of HCV-related liver disease, but this invasive procedure is not without risk[1]. There is a low mortality rate but a high error rate, predominantly owing to undersampling, whereby typically, less than 1/50000 of the liver volume is obtained for histological evaluation[2-5]. These factors highlight the need for a noninvasive test to characterise diffuse liver disease.
For ethical reasons and because most patients are unwilling to undergo repeated procedures, treatment algorithms rarely allow serial liver biopsy. Thus, the impetus to find a reliable and repeatable biomarker of disease activity and response to treatment has a renewed focus[6].
Clinical (in vivo) phosphorus-31 magnetic resonance spectroscopy (31P MRS) is the only noninvasive technique that can be used to provide direct localised biochemical information on hepatic metabolic processes. A typical 31P MR spectrum of the human liver in vivo contains resonances that can be assigned to phosphomonoesters (PMEs), containing information from sugar phosphates in the glycolytic pathway and from cell membrane precursors such as phosphoethanolamine and phosphocholine; and to phosphodiesters[7], containing information from the endoplasmic reticulum and from cell membrane degradation products such as glycerophosphorylcholine and glycerophosphorylethanolamine, in addition to signals from inorganic phosphate and nucleotide triphosphates, including adenosine triphosphate. Many studies have reported a good correlation between elevated PME resonance and decreased phosphodiester (PDE) resonance in cirrhosis[8-10]. The ratio of PME to PDE has traditionally been viewed as an index of cell membrane turnover and thus provides an indirect measure of grading of liver histology[9].
The aim of the current study was to investigate the utility of 31P MRS as a noninvasive test for assessment of response to interferon and ribavirin treatment in patients with different severities of HCV.
From January 2010 to June 2010, 120 patients with chronic hepatitis C were enrolled. The diagnosis of decompensated HCV-induced cirrhosis was based on the American Association for the Study of Liver Diseases Clinical Guideline for Hepatitis C (2004).
All enrolled patients were naive to antiviral treatments. Other inclusion criteria were: (1) HCV RNA >500 copies/mL; (2) absence of complications such as gastrointestinal bleeding, hepatic encephalopathy, and primary liver cancer; and (3) liver function defined as Child-Pugh grade B or C based on serum bilirubin, serum albumin, presence of ascites, presence of hepatic encephalopathy, and prothrombin time. Patients with hypersplenism were also enrolled. Exclusion criteria were: (1) infection with hepatitis A, B, D, or F virus, Epstein-Barr virus, cytomegalovirus, or human immunodeficiency virus; and (2) presence of alcoholic or drug-induced liver diseases, or severe heart, brain, or kidney disease.
A total of 120 patients meeting the inclusion criteria were enrolled. Patients were considered as part of the treatment group (n = 90) or control group (n = 30), based on whether they opted to receive antiviral therapy. The study was approved by the Institutional Review Board of the hospital, and informed consent was obtained from all study participants.
Determination of therapeutic efficacy: The primary endpoints were: (1) SVR, defined as HCV RNA undetectable or < 500 copies/mL for at least 24 wk after treatment discontinuation[11]; and (2) relapse, defined as HCV RNA undetectable or < 500 copies/mL during antiviral therapy, but becomes detectable at 24 wk after treatment discontinuation. The secondary endpoints were disease progression (defined as an increase of 2 or more in the Child-Pugh score), presence of primary hepatocellular carcinoma, renal dysfunction, spontaneous bacterial peritonitis, variceal bleeding, or death due to liver disease[12].
Measures: Patients in the treatment group were evaluated for serum HCV antibodies, liver function, HCV RNA, coagulation function, thyroid function, and alpha foetoprotein as well as liver computed tomography. Routine blood and urine tests were performed before the start of the study. Routine blood and liver function tests were performed weekly in the first month, then once every 4 wk during the study period and once every 8 wk for 24 wk after discontinuation of treatment. Quantitative detection of HCV RNA was done immediately prior to treatment (baseline), at 24 and 48 wk after treatment, and 6 mo after discontinuation of treatment. HCV RNA levels were quantitated by real-time polymerase chain reaction using a kit from the Roche company.
Patients in the control group were evaluated for liver function and HCV RNA levels. Routine blood tests and colour ultrasonography of the liver were done every 12 wk. All patients were assessed for disease progression.
Treatment regimen and follow-up: All participants received symptomatic and supportive treatment, including treatment for reducing levels of transaminase and bilirubin and supplemental albumin. For patients in the treatment group, those who had a neutrophil count ≥ 1.0 × 109/L, platelet count ≥ 50 × 109/L, and haemoglobin > 10 g/L were treated additionally with both pegylated interferon α 2a (Peg-IFNα-2a) and ribavirin (RBV). The initial dose of Peg-IFNα-2a was 180 μg/kg subcutaneously. Peg-IFNα-2a dosage was reduced to 90 μg/kg once weekly when neutrophil or platelet counts decreased to ≤ 0.75 × 109/L or < 50 × 109/L, respectively. The dose was returned to 180 μg/kg if neutrophil and platelet counts increased to > 0.75 × 109/L and ≥ 50 × 109/L, respectively, after 2 wk. Treatment was discontinued if neutrophil count was ≤ 0.5 × 109/L or platelet count was < 30 × 109/L. Patients tolerating the standard Peg-IFNα-2a dose of 180 μg/kg weekly were treated for 48 weeks. Patients who could not tolerate the standard dose were treated with the reduced dose of 90 μg/kg once weekly for up to 72 wk.
Patients with haemoglobin >100 g/L were initially treated with a standard dose of RBV (genotype 1: 1200 mg/d for patients with body weight > 75 kg and 1000 mg/d for patients with body weight ≤ 75 kg; non-genotype 1: 1000 mg/d for patients with body weight >75 kg and 800 mg/d for patients with body weight ≤ 75 kg). RBV dosage was reduced when haemoglobin levels decreased to ≤ 100g/L after the dosage increase. RBV treatment was discontinued when haemoglobin levels were ≤ 80 g/L. Patients tolerating the standard dose of RBV were treated for 48 wk. Patients developing cytopaenia during the treatment period were treated with cell growth-stimulating factor and/or erythropoietin. All patients were followed for 3 years.
A 3.0T MRI unit (Philips Medical Systems) was used[6]. All imaging was conducted after an overnight fast. An enveloping transmitter coil and a separate surface receiver coil were used. Both coils were double-tuned for protons at 64 MHz and phosphorus at 26 MHz. The proton signal was used to obtain a T1-weighted image (TR/TE, 800/16) in the axial plane to confirm patient positioning. The 31P MR spectra were localised to a centrally placed voxel within the liver by use of an image-selected in vivo spectroscopy sequence (voxel size, 70 mm × 70 mm × 70 mm; TR, 10000; number of signals averaged, 48). A voxel location within the right liver away from major vessels was used for each patient and was consistent for all baseline and follow-up images. The total examination time was 40 min with a 10-min acquisition time for the 31P MRS sequences. All patients underwent baseline 31P MRS before the start of antiviral treatment, and all underwent follow-up imaging 6 mo after the start of treatment.
Quantitation of the 31P signals was performed in the time domain with the advanced method for accurate, robust, and efficient spectral fitting (AMARES) algorithm included in the Magnetic Resonance User Interface (MRUI) software program (http://www.mrui.uab.es/mrui). Anonymity was assured and MR spectra were analysed by one blinded observer. The spectra were rechecked by another blinded observer. Peak areas for PME, PDE, inorganic phosphate, and the three nucleoside triphosphate moieties (γ, α, and β) were obtained with respect to the total phosphorus signal intensity. Because of previous findings highlighting the utility of the PME/PDE ratio, this index was used for further statistical analysis. Data from a bank of 15 age-matched healthy volunteers without a history of liver disease were used for comparison.
Age and baseline HCV RNA levels were normally distributed and presented as mean and standard deviation. Differences in age and baseline HCV RNA levels between the two groups were tested by the independent two-sample t-test. Child-Pugh scores were non-normally distributed and are presented as median and inter-quartile range. Differences in Child-Pugh scores between the two groups were tested by the non-parametric Mann-Whitney test. Other categorical variables are presented as number and percentage, and categorical variables were compared using the Fisher’s exact test. Statistically significant variables from the univariate analyses were used in the multivariate analysis. All statistical tests were two-sided, and a P-value < 0.05 was considered statistically significant. All statistical analyses were performed using the SPSS 19.0 software (SPSS Inc, Chicago, IL, United States).
As shown in Table 1, 120 patients who met the inclusion criteria were enrolled. Among them, 90 patients had sufficient blood cell counts for antiviral therapy. The remaining 30 patients, who refused antiviral therapy, were placed in the control group. Patients in the treatment group were significantly younger than those in the control group (mean age 52.7 vs 58.3 years, respectively, P < 0.001). There were no significant differences between the two groups in baseline HCV RNA levels. In addition, baseline MELD scores were not significantly different between the treatment and control groups (Table 1). Although baseline Child-Pugh scores, total bilirubin, and hepatic encephalopathy were not different between the two groups, significant differences in serum albumin, international normalised ratio (INR) for prothrombin time, and ascites were observed between the treatment and control groups (P = 0.002, P = 0.018, and P < 0.001, respectively).
| Treatment (n = 90) | Control (n = 30) | P-value | |
| Age (yr) | 52.7 ± 10.1 | 58.3 ± 12.5 | < 0.0011 |
| Gender | |||
| Male | 36 (40.0) | 14 (46.7) | 0.573 |
| Female | 54 (60.0) | 16 (53.3) | |
| Baseline HCV RNA level (log10 copies/mL) | 5.30 ± 1.18 | 5.23 ± 1.15 | 0.681 |
| Baseline MELD score | 12.6 (9.8, 15.2) | 12.5 (9.4, 15.8) | 0.654 |
| Baseline Child-Pugh score | 9.0 (7.0, 10.0) | 8.0 (7.0, 10.0) | 0.809 |
| Total bilirubin (mg/dL) | |||
| < 2 | 9 (10.0) | 5 (16.67) | 0.691 |
| 2-3 | 40 (44.4) | 12 (40.0) | |
| > 3 | 41 (45.6) | 13 (43.33) | |
| Serum albumin (g/dL) | |||
| > 3.5 | 9 (10.0) | 3 (10.0) | 0.0051 |
| 2.8-3.5 | 40 (44.4) | 19 (63.3) | |
| < 2.8 | 41 (45.6) | 8 (26.7) | |
| Prothrombin time INR | |||
| < 1.7 | 26 (28.9) | 8 (26.7) | 0.0291 |
| 1.7-2.3 | 50 (55.6) | 13 (43.3) | |
| > 2.3 | 14 (15.5) | 9 (30.0) | |
| Hepatic encephalopathy | |||
| None | 90 (100.0) | 30 (100.0) | NA |
| Ascites | |||
| Absent | 90 (100.0) | 26 (87.4) | < 0.0011 |
| Easily controlled | 0 (0.0) | 4 (13.3) |
The PME/PDE ratios at 6 mo after the start of antiviral therapy in the Child B and C groups were significantly higher than those before therapy, but this was not seen in Child-Pugh A group (Table 2).
| Child A | Child B | Child C | |
| Before therapy | 0.20 ± 0.17 | 0.27 ± 0.24 | 0.39 ± 0.18 |
| Six mo after the start of therapy | 0.16 ± 0.09 | 0.19 ± 0.12 | 0.22 ± 0.16 |
| P | > 0.05 | < 0.05 | < 0.05 |
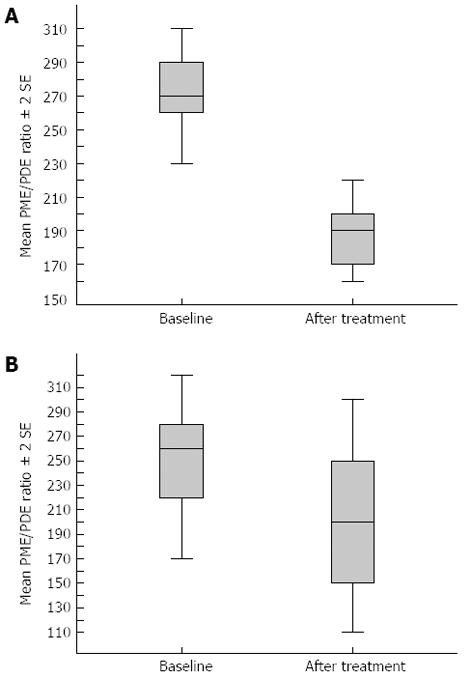
Sixty-nine patients responded to antiviral treatment with a sustained viral response. In 54 of these patients, the PME/PDE ratio had decreased toward normal on follow-up MRS. Figure 1 is the graph of a responder whose spectra changed after treatment, showing a decrease in PME/PDE ratio. Fifteen of the 21 virological nonresponders had PME/PDE ratios on follow-up imaging similar to the baseline values. Another two nonresponders had an increase in the PME/PDE ratio on follow-up imaging (Table 3). An unchanged PME/PDE ratio was defined as a difference of not more than 0.03 in comparison with the baseline ratio. An increase was defined as a > 0.03 increase in PME/PDE ratio in comparison with the baseline value. A decrease in PME/PDE ratio was defined as a > 0.03 reduction in the ratio compared with the baseline value.
| Patient group | PME/PDE decreased | PME/PDE unchanged | PME/PDE increased |
| Responders (n = 69) | 54 (78) | 9 (13) | 6 (9) |
| Nonresponders (n = 21) | 2 (10) | 4 (20) | 15 (70) |
| P | > 0.05 | < 0.05 | < 0.05 |
It is estimated that approximately 3% of the global population has chronic infection with the HCV and that approximately 4 million persons are newly infected each year[13]. In 55%-85% of patients, the infection develops into chronic liver disease, which in many cases remains asymptomatic. In approximately 20% of cases, fibrosis develops into cirrhosis, which leads to hepatocellular cancer in 5% of cases each year[14]. Liver biopsy is the reference standard for staging and grading chronic liver disease, but this invasive procedure is not without risk. There is a low mortality rate but a high error rate, predominantly owing to undersampling, whereby typically, less than 1/50000 of the liver volume is obtained for histological evaluation[2,15]. As a result of the problems associated with biopsy, a steady drive to find an effective noninvasive method for evaluating liver damage has led to developments both in testing with serological biomarkers of disease and in imaging. For ethical reasons and because most patients are unwilling to undergo repeated procedures, treatment algorithms in the United Kingdom rarely allow serial liver biopsy. Thus, the impetus to find a reliable and repeatable biomarker of disease activity and response to treatment has a renewed focus[6].
One particular noninvasive technique for characterising chronic liver disease is 31P MRS. Clinical (in vivo) 31P MRS is a noninvasive technique that can be used to provide direct localised biochemical information on hepatic metabolic processes. At present, many reports suggest that there is a clear correlation between 31P MR spectral classification and liver disease jurisprudence[6]. However, because of the sensitivity and specificity, especially for chronic hepatitis C patients, it is needed to monitor changes of liver histology after antiviral treatment.
A typical 31P MR spectrum of the human liver in vivo contains resonances that can be assigned to PMEs, containing information from sugar phosphates in the glycolytic pathway and from cell membrane precursors such as phosphoethanolamine and phosphocholine; and to PDEs[7], containing information from the endoplasmic reticulum and from cell membrane degradation products such as glycerophosphorylcholine and glycerophosphorylethanolamine. In addition, in patients with precirrhotic liver disease, 31P MRS can be used in grading disease severity and compared with histology from liver biopsy. Research reports that 31P MRS PME is elevated in patients with cirrhosis and PDE is reduced. Thus, the PME/PDE ratio can be used as an indirect sign of liver disease at the metabolic level[16]. Some studies suggested that PME/PDE ratio increased with increasing severity of chronic liver disease and that this ratio was highly sensitive for the presence of cirrhosis[17]. With noninvasive imaging, we used the PME/PDE ratio to assess the severity of precirrhotic HCV-related liver disease[14].
In this study, the PME/PDE ratio was significantly decreased in the sustained virological responder group. This ratio remained the same or was increased in patients who were virological nonresponders (Figure 1). PME resonance contains contributions from cell membrane precursors and PDE resonance contains contributions from cell membrane degradation products[18,19]. The PME/PDE ratio thus gives information on cell turnover within the liver[20]. It has been shown that this ratio is reduced after effective viral eradication treatment[21,22]. It is also of interest that cirrhosis patients of the responders group also had a reduction in the PME/PDE ratio. Study findings of a good correlation between the PME/PDE ratio and degree of liver fibrosis[6] suggest that liver fibrosis can regress in patients with cirrhosis. The number of patients in our sample was too small for an absolute conclusion, but the findings fuel this controversial area. Overall, the results show that 31P MRS can be used as a completely noninvasive imaging indicator of response to treatment in a population of patients who may be undergoing imaging anyway, that is, patients with established cirrhosis undergoing screening for the development of hepatocellular carcinoma.
31P PME/PDE ratio is not 100% sensitive or specific. In our study, some patients who did not have a sustained response had a reduction in the PME/PDE ratio. Similarly, some patients in the sustained virological responder group had a worsening PME/PDE ratio but were subsequently found to be clear of the virus in longer-term virological follow-up studies. The PME/PDE ratios we obtained were at baseline and 6 mo after the start of antiviral therapy, but most patients should continue antiviral therapy for over 1 year, so repeating examination with 31P PME/PDE may bring higher sensitivity or specificity. On the other hand, the PME/PDE ratio could provide biochemical information on hepatic metabolic processes, which could indicate resolution of fibrosis.
This study indicates that the PME/PDE ratio can be used as an indicator of response to treatment. Most modern MR systems have the capability for MRS. 31P MRS is a noninvasive technique that can be used to provide direct localised biochemical information on hepatic metabolic processes. It is a useful technique for chronic hepatitis C patients on antiviral therapy.
Hepatitis C virus (HCV) is one of the leading causes of liver disease worldwide. Liver biopsy remains the gold standard for providing the stage (extent of fibrosis) and grade (degree of NI activity) of HCV-related liver disease, but this invasive procedure is not without risk. Thus, the impetus to find a reliable and repeatable biomarker of disease activity and response to treatment has a renewed focus.
Clinical (in vivo) phosphorus-31 magnetic resonance spectroscopy (31P MRS) is the only noninvasive technique that can be used to provide direct localised biochemical information on hepatic metabolic processes.
This study was the first attempt to use 3.0T 31P MRS in assessment of response to antiviral therapy for chronic hepatitis C. It assessed the value of 3.0T 31P MRS, a noninvasive technique, in testing response to antiviral therapy for chronic hepatitis C. The technique could provide biochemical information on hepatic metabolic processes. The phosphomonoester (PME)/phosphodiester (PDE) ratio can be used as an indicator of response to antiviral treatment in chronic hepatitis C patients.
This study suggests that 31P MRS could provide biochemical information on hepatic metabolic processes. The PME/PDE ratio can be used as an indicator of response to antiviral treatment in chronic hepatitis C patients.
Clinical (in vivo) 31P MRS is the only noninvasive technique that can be used to provide direct localised biochemical information on hepatic metabolic processes. A typical 31PMR spectrum of the human liver in vivo contains resonances that can be assigned to PMEs, containing information from sugar phosphates in the glycolytic pathway and from cell membrane precursors such as phosphoethanolamine and phosphocholine; and to PDEs, containing information from the endoplasmic reticulum and from cell membrane degradation products such as glycerophosphorylcholine and glycerophosphorylethanolamine, in addition to signals from inorganic phosphate and nucleotide triphosphates, including adenosine triphosphate. Many studies have reported a good correlation between elevated PME resonance and decreased PDE resonance in cirrhosis. The ratio of PME to PDE has traditionally been viewed as an index of cell membrane turnover and thus provides an indirect measure of grading of liver histology.
This is a good descriptive study in which authors attempt to use 3.0T 31P MRS in assessment of response to antiviral therapy for chronic hepatitis C. 3.0T 31P MRS represents a new noninvasive technique that provides biochemical information on hepatic metabolic processes and response to antiviral therapy for chronic hepatitis C.
P- Reviewers: Asselah T, Salami A S- Editor: Wang JL L- Editor: Wang TQ E- Editor: Zhang DN
| 1. | Fontana RJ, Lok AS. Noninvasive monitoring of patients with chronic hepatitis C. Hepatology. 2002;36:S57-S64. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 21] [Cited by in F6Publishing: 48] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 2. | Regev A, Berho M, Jeffers LJ, Milikowski C, Molina EG, Pyrsopoulos NT, Feng ZZ, Reddy KR, Schiff ER. Sampling error and intraobserver variation in liver biopsy in patients with chronic HCV infection. Am J Gastroenterol. 2002;97:2614-2618. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 1504] [Cited by in F6Publishing: 1504] [Article Influence: 68.4] [Reference Citation Analysis (0)] |
| 3. | Colloredo G, Guido M, Sonzogni A, Leandro G. Impact of liver biopsy size on histological evaluation of chronic viral hepatitis: the smaller the sample, the milder the disease. J Hepatol. 2003;39:239-244. [PubMed] [Cited in This Article: ] |
| 4. | Bedossa P, Poynard T. An algorithm for the grading of activity in chronic hepatitis C. The METAVIR Cooperative Study Group. Hepatology. 1996;24:289-293. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 2860] [Cited by in F6Publishing: 2932] [Article Influence: 104.7] [Reference Citation Analysis (0)] |
| 5. | Bedossa P, Dargère D, Paradis V. Sampling variability of liver fibrosis in chronic hepatitis C. Hepatology. 2003;38:1449-1457. [PubMed] [Cited in This Article: ] |
| 6. | Cobbold J, Lim A, Wylezinska M, Cunningham C, Crossey M, Thomas H, Patel N, Cox J, Taylor-Robinson S. Magnetic resonance and ultrasound techniques for the evaluation of hepatic fibrosis. Hepatology. 2006;43:1401-142; author reply 1402. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 10] [Cited by in F6Publishing: 12] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 7. | Mariana VF, de Fátima GG, Maria Pde G. The effect of mechanical lymph drainage accompanied with heat on lymphedema. J Res Med Sci. 2011;16:1448-1451. [PubMed] [Cited in This Article: ] |
| 8. | Menon DK, Sargentoni J, Taylor-Robinson SD, Bell JD, Cox IJ, Bryant DJ, Coutts GA, Rolles K, Burroughs AK, Morgan MY. Effect of functional grade and etiology on in vivo hepatic phosphorus-31 magnetic resonance spectroscopy in cirrhosis: biochemical basis of spectral appearances. Hepatology. 1995;21:417-427. [PubMed] [Cited in This Article: ] |
| 9. | Lim AK, Patel N, Hamilton G, Hajnal JV, Goldin RD, Taylor-Robinson SD. The relationship of in vivo 31P MR spectroscopy to histology in chronic hepatitis C. Hepatology. 2003;37:788-794. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 97] [Cited by in F6Publishing: 102] [Article Influence: 4.9] [Reference Citation Analysis (0)] |
| 10. | Lim AK, Patel N, Hamilton G, Mylvahan K, Kuo YT, Goldin RD, Taylor-Robinson SD. 31P MR spectroscopy in assessment of response to antiviral therapy for hepatitis C virus-related liver disease. AJR Am J Roentgenol. 2007;189:819-823. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 13] [Cited by in F6Publishing: 15] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 11. | Pearlman BL. Protease inhibitors for the treatment of chronic hepatitis C genotype-1 infection: the new standard of care. Lancet Infect Dis. 2012;12:717-728. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 89] [Cited by in F6Publishing: 92] [Article Influence: 7.7] [Reference Citation Analysis (0)] |
| 12. | Liaw YF, Sung JJ, Chow WC, Farrell G, Lee CZ, Yuen H, Tanwandee T, Tao QM, Shue K, Keene ON, Dixon JS, Gray DF, Sabbat J. Lamivudine for patients with chronic hepatitis B and advanced liver disease. N Engl J Med. 2004;351:1521-1531. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 1739] [Cited by in F6Publishing: 1648] [Article Influence: 82.4] [Reference Citation Analysis (0)] |
| 13. | Hoofnagle JH. Course and outcome of hepatitis C. Hepatology. 2002;36:S21-S29. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 104] [Cited by in F6Publishing: 361] [Article Influence: 16.4] [Reference Citation Analysis (0)] |
| 14. | Brook G, Soriano V, Bergin C. European guideline for the management of hepatitis B and C virus infections, 2010. Int J STD AIDS. 2010;21:669-678. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 16] [Cited by in F6Publishing: 18] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 15. | Ishak K, Baptista A, Bianchi L, Callea F, De Groote J, Gudat F, Denk H, Desmet V, Korb G, MacSween RN. Histological grading and staging of chronic hepatitis. J Hepatol. 1995;22:696-699. [PubMed] [Cited in This Article: ] |
| 16. | Auricchio A, Zhou R, Wilson JM, Glickson JD. In vivo detection of gene expression in liver by 31P nuclear magnetic resonance spectroscopy employing creatine kinase as a marker gene. Proc Natl Acad Sci USA. 2001;98:5205-5210. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 43] [Cited by in F6Publishing: 45] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 17. | Schuhmann MU, Stiller D, Skardelly M, Bernarding J, Klinge PM, Samii A, Samii M, Brinker T. Metabolic changes in the vicinity of brain contusions: a proton magnetic resonance spectroscopy and histology study. J Neurotrauma. 2003;20:725-743. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 84] [Cited by in F6Publishing: 86] [Article Influence: 4.1] [Reference Citation Analysis (0)] |
| 18. | Taylor-Robinson SD, Sargentoni J, Bell JD, Thomas EL, Marcus CD, Changani KK, Saeed N, Hodgson HJ, Davidson BR, Burroughs AK. In vivo and in vitro hepatic phosphorus-31 magnetic resonance spectroscopy and electron microscopy in chronic ductopenic rejection of human liver allografts. Gut. 1998;42:735-743. [PubMed] [Cited in This Article: ] |
| 19. | Taylor-Robinson SD, Thomas EL, Sargentoni J, Marcus CD, Davidson BR, Bell JD. Cirrhosis of the human liver: an in vitro 31P nuclear magnetic resonance study. Biochim Biophys Acta. 1995;1272:113-118. [PubMed] [Cited in This Article: ] |
| 20. | Tan SL, Pause A, Shi Y, Sonenberg N. Hepatitis C therapeutics: current status and emerging strategies. Nat Rev Drug Discov. 2002;1:867-881. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 164] [Cited by in F6Publishing: 168] [Article Influence: 7.6] [Reference Citation Analysis (0)] |
| 21. | Taylor-Robinson SD, Sargentoni J, Bell JD, Saeed N, Changani KK, Davidson BR, Rolles K, Burroughs AK, Hodgson HJ, Foster CS. In vivo and in vitro hepatic 31P magnetic resonance spectroscopy and electron microscopy of the cirrhotic liver. Liver. 1997;17:198-209. [PubMed] [Cited in This Article: ] |
| 22. | Kiyono K, Shibata A, Sone S, Watanabe T, Oguchi M, Shikama N, Ichijo T, Kiyosawa K, Sodeyama T. Relationship of 31P MR spectroscopy to the histopathological grading of chronic hepatitis and response to therapy. Acta Radiol. 1998;39:309-314. [PubMed] [Cited in This Article: ] |