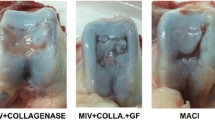
Abstract
The effectiveness of autologous rib perichondrium for repair of full-thickness hyaline cartilage defects has been shown experimentally and clinically in various reports. The purpose of this study was to examine the behaviour of sheep rib perichondrial tissue under in vitro conditions and the influence of different culture matrices in order to evaluate possible stimulating effects. Rib perichondrium was obtained from sheep used for an experimental in vivo trial. After removal of adjacent cartilage remnants the tissue was devided and specimens cultured for 14 days in different ways. Explants cultured on collagen sponges (group A), fibrin glue (group B) and cellulose acetate filter (group C) were examined histologically, histochemically, histomorphometrically and autoradiographically. Clear differentiation of perichondrial cells towards a chondrocyte-like cell shape, particularly in the proliferation zone, was noticed on all matrices. These cells synthesized new matrix substances comparable to the ground substance normally present in hyaline cartilage. Morphometric comparison of tissue differentiation on different culture matrices revealed no significant differences in proliferation rates.
Similar content being viewed by others
References
Amiel D, Harwood FL, Abel MF, Akeson WH (1985a) Collagen types in neo-cartilage tissue resulting from rip perichondrial graft in an articular defect-a rapid semi-quantitative methodology. Collagen Relat Res 5:337–347
Amiel D, Coutts RD, Abel M, Stewart W, Harwood F, Akeson WH (1985b) Rib perichondrial grafts for the repair of full-thickness articular cartilage defects. J Bone Joint Surg [Am] 67:911–920
Amiel D, Coutts RD, Harwood FL, Ishizue KK, Kleiner JB (1988) The chondrogenesis of rib perichondrial grafts for repair of full-thickness articular cartilage defects in a rabbit model: a one year postoperative assessment. Connect Tissue Res 18:27–39
Bentley G (1978) The surgical treatment of chondromalacia patellae. J Bone Joint Surg [Br] 60:74–81
Bruns J, Kersten P, Lierse W, Silbermann M (1992a) Autologous rib perichondrial grafts in experimentally induced osteochondral lesions in the sheep-knee joint: morphological results. Virchos Archiv [A] 421:1–8
Bruns J, Meyer-Pannwitt U, Silbermann M (1992b) The rib perichondrium. An anatomical study in sheep of a tissue used as transplant in the treatment of hyaline-cartilage defects. Acta Anat [Basel] 144:258–266
Bulstra SK, Homminga GN, Buurman WA, Terwindt-Rouwenhorst E, Linden AJ van der (1990) The potential of adult human perichondrium to form hyaline cartilage in vitro. J Orthop Res 8:328–335
Calandruccio RA, Gilmer WS (1962) Proliferation, regeneration, and repair of articular cartilage of immature animals. J Bone Joint Surg [Am] 44:431–455
Carrino DA, Lennon DP, Caplan AI (1983) Extracellular matrix and the maintenance of the differentiated state: proteoglycans synthesized by replanted chondrocytes and nonchondrocytes. Dev Biol 99:132–144
Doerner (1798) De gravioribus quibusdam cartilaginum mutationibus. Tubingae. Cited by Mori M (1905) Studien über Knorpelregeneration. Dtsch Z Chir 76:220–234
Engkvist O (1979) Reconstruction of patellar articular cartilage with free autologous perichondrial grafts (in dogs). Scand J Plast Reconstr Surg 13:361–369
Engkvist O, Johansson SH (1980) Perichondrial arthroplasty. Scand J Plast Reconstr Surg 14:71–87
Engkvist O, Ohlsen L (1979) Reconstruction of articular cartilage with free autologous perichondral grafts. Scand J Plast Reconstr Surg 13:269–274
Engkvist O, Skoog V, Pastacaldi P, Yormuk E, Juhlin R (1979) The cartilaginous potential of the perichondrium in rabbit ear and rib. Scand J Plast Reconstr Surg 13:275–280
Gaudernak T, Zifko B, Skorpik G (1986) Clinical experiences using fibrin sealant in the treatment of osteochondral fractures. In: Schlag G, Redl H (eds) Fibrin sealant in operative medicine, vol 7. Traumatology — Orthopaedics. Springer, Berlin Heidelberg New York, pp 91–102
Gluecksman A (1939) Studies on bone mechanics in vitro. II. The role of tension and pressure in chondrogenesis. Anat Rec 73:39–55
Homminga GN, Linden TJ van der, Terwindt-Rouwenhorst EAW (1989) Repair of articular defects by perichondrial grafts. Acta Orthop Scand 60:326–329
Homminga GN, Bulstra SK, Bouwmeester PM, Linden AJ van der (1990) Perichondrial grafting for cartilage lesions of the knee. J Bone Joint Surg [Br] 72:1003–1007
Itay S, Abramovici A, Nevo Z (1987) The use of cultured embryonal chondrocytes as grafts for defects created in articular cartilage. Clin Orthop 220:270–279
Johnson LL (1986) Arthroscopic abrasion arthroplasty historical and pathologic perspective: present status. Arthroscopy 2:54–69
Kaplonyi G, Zimmerman I, Frenyo AD, Farkas T, Nemes G (1988) The use of fibrin adhesive in the repair of chondral and osteochondral injuries. Injury 19:267–272
Kato Y, Watanabe R, Tsuji M, Suzuki F, Canalis E (1984) Effect of bone-derived growth factor on DNA, RNA, and proteoglycan synthesis in cultures of rabbit costal chondrocytes. Metabolism 31:812–816
Kimura T, Yasui N, Ohsawa S, Ono K (1984) Chondrocytes embedded in collagen gels maintain cartilage phenotype during long-term cultures. Clin Orthop 186:231–239
Kiviranta I, Tammi M, Jurvelin J, Säämänen AM, Helminen HJ (1985) Demonstration of chondroitin sulphate and glycoproteins in articular cartilage matrix using periodic acid-Schiff (PAS) method. Histochemistry 83:303–306
Kon M (1981) Cartilage formation from perichondrium in a weight-bearing joint. Eur Surg Res 13:387–396
Kosher RA, Lash JW, Minor RR (1973) Environmental enhancement of in vitro chondrogenesis. IV. Stimulation of somite chondrogenesis by exogenous chondromucoprotein. Dev Biol 35:210–220
Maor G, Mark K VD, Reddi H, Heinegard D, Franzen A, Silbermann M (1987) Acceleration of cartilage and bone formation on collagenous substrata. Collagen Relat Res 7:351–370
Maruyama Y (1979) An experimental study on cartilage formation in autogenous perichondrial transplantation in rabbits. Keio J Med 28:63–72
Meachim G, Roberts C (1971) Repair of the joint surface from subarticular tissue in the rabbit knee. J Anat 109:317–327
Mitchell N, Shepard N (1980) Healing of articular cartilage in intra-articular fractures in rabbits. J Bone Joint Surg [Am] 62:628–634
Nelson BH, Anderson DD, Brand RA, Brown TD (1988) Effect of osteochondral defects on articular cartilage. Acta Orthop Scand 59:574–579
Nevo Z, Dorfman A (1972) Stimulation of chondromucoprotein synthesis in chondrocytes by extracellular chondromucoprotein. Proc Natl Acad Sci USA 69:2069–2072
Nevo Z, Horwitz AL, Dorfman A (1972) Synthesis of chondromucoprotein by chondrocytes in suspension culture. Dev Biol 28:219–228
Niedermann B, Boe S, Lauritzen J, Rubak JM (1985) Glued periosteal grafts in the knee. Acta Orthop Scand 56:457–460
Pastacaldi P, Engkvist O (1979) Perichondrial wrist arthroplasty in rheumatoid patients. Hand 11:184–190
Ritsilä V, Poussa M, Rubak J, Snellman O, Österman K (1980) Periosteal and perichondral grafts in reconstruction of the patellar joint surface. Acta Orthop Scand 51:704
Ritsilä V, Poussa M, Rubak J, Snellman O, Österman K (1981) Periosteal and perichondrial grafts in reconstruction of joint surfaces. Acta Orthop Scand 52:447
Sakai A, Suzuki K, Nakamura T, Norimura T, Tsuchiya T (1991) Effect of pulsing electromagnetic fields on cultured cartilage cells. Int Orthop 15:341–346
Schwartz NB, Dorfman A (1975) Stimulation of chondroitin sulfate proteoglycan production by chondrocytes in monolayer. Connect Tissue Res 3:115–122
Serradge H, Kutz JA, Kleinert HE, Lister GD, Wolff TW, Atasoy E (1984) Perichondrial resurfacing arthroplasty in the hand. J Hand Surg [A] 9:880–886
Silbermann M, Frommer J (1974) Demonstration and distribution of acidic glycosaminoglycans in mouse secondary cartilage. Histochemistry 38:85–93
Silbermann M, Kadar T, Hornung G (1977) Corticoid-induced changes in glucose metabolism of chondrocytes. Histochemistry 50:327–335
Skoog T, Ohlsen L, Sohn SA (1972) Perichondrial potential for cartilagenous regeneration. Scand J Plast Reconstr Surg 6:123–125
Skoog T, Ohlsen L, Sohn SA (1975) The chondrogenic potential of the perichondrium. Chir Plastica 3:91–103
Solursh M, Meier S (1974) Effects of cell density of the expression of differentiation by chick embryo chondrocytes. J Exp Zool 187:311–322
Sully L, Jackson IT, Sommerland BC (1980) Perichondrial grafting in rheumatoid metacarpophalangeal joints. Hand 12:137–148
Tajima S, Aoyagi F, Maruyama Y (1978) Free perichondrial grafting in the treatment of temporomandibular joint ankylosis. Plast Reconstr Surg 61:876–880
Tiling T (1986) Follow-up after reattachment of chondral and osteochondral fragments of the knee joint. In: Schlag G, Redl H (eds) Fibrin sealant in operative medicine, vol 7. Traumatology — orthopaedics. Springer, Berlin Heidelberg New York, pp 74–78
Tizzoni G (1878) Sulla istologia normale e patologica delle cartilagini ialine. Arch Sci Med 2:27–102
Upton J, Sohn SA, Glowacki J (1981) Neocartilage derived from transplanted perichondrium: what is it? Plast Reconstr Surg 68:166–172
Veldhuijzen JP, Bourret LA, Rodan L (1979) In vitro studies of the effect of intermittent compressive forces on cartilage cell proliferation. J Cell Physiol 98:299–306
Wakitani S, Kimura T, Hirooka A, Ochi T, Yoneda M, Yasui N, Owaki H, Ono K (1989) Repair of rabbit articular surfaces with allograft chondrocytes embedded in collagen gel. J Bone Joint Surg [Br] 71:74–80
Woo SL-Y, Kwan M, Lee TQ, Field FP, Kleiner JB, Coutts RD (1987) Perichondrial autograft for the articular cartilage. Shear modulus of neocartilage studied in rabbits. Acta Orthop Scand 58:510–515
Yasui N, Osawa S, Ochi T, Nakashima H, Ono K (1982) Primary culture of chondrocytes embedded in collagen gels. Exp Cell Biol 50:92–100
Zilch H (1986) Glueing of osteochondral fragments and fixation of dissecates in osteochondrosis dissecans. In: Schlag G, Redl H (eds) Fibrin sealant in operative medicine, vol 7. Traumatology — orthopaedics. Springer, Berlin Heidelberg New York, pp 63–73
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Bruns, J., Kersten, P., Weiss, A. et al. The in vitro influence of different culture conditions on the potential of sheep rib perichondrium to form hyaline-like cartilage. Vichows Archiv A Pathol Anat 424, 169–175 (1994). https://doi.org/10.1007/BF00193497
Received:
Accepted:
Issue Date:
DOI: https://doi.org/10.1007/BF00193497