Summary
The present study provides a LM and EM inventory of the fibers of the rat abdominal vagus, including dorsal and ventral trunks and the five primary branches. Whole mounts (n = 15) were prepared to characterize the branching patterns. A set of EM samples consisting of both trunks and all branches (i.e. dorsal and ventral gastric, dorsal and accessory celiac, and hepatic) were then obtained from each of six additional animals. A complete cross-sectional montage (x 10000) was prepared from each sample. All axons were counted, and >10% of them were evaluated morphometrically.
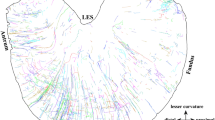
The means of unmyelinated axon diameters for each of the five branches were similar (0.75–0.83 μm). However, the shapes of the fiber size distributions, as summarized by their skew coefficients, revealed that the two gastric branches differed significantly from the two celiac branches; furthermore, the hepatic size distribution differed from all others. Most of the myelinated fibers (85%) in all branches were <2.6 μm in diameter and had sheath widths between 0.1 and 0.5 μm. The gastric branches, however, also contained a few larger myelinated fibers with sheath widths as great as 0.85 μm. Whole mounts revealed fibers which were not of supradiaphragmatic orgin within all five vagal branches; these adventitial bundles were traced along the perineurium between adjacent branches. The sum of the fibers in the five branches (26930) was 21% more than the number counted in the parent trunks (22272); this excess probably reflects the adventitial fiber content. The whole mounts also showed that a large and regularly positioned paraganglion was associated with the dorsal branches.
The structural profiles observed (i.e. unmyelinated and myelinated fibers size distributions, presence of extrinsic fascicles, glomus tissue content, etc.) differentiate the vagal branches into three morphologically distinct sets: a gastric pair, a celiac pair, and a hepatic branch. The fiber counts, when considered with observations of the numbers of efferents and adventitial fibers in the nerve, suggest that the percentage of efferent fibers is much higher than in all the widely accepted estimates found in the literature: efferent fibers may represent over a quarter of the total number of fibers.
Similar content being viewed by others
References
Agostoni E, Chinnock JE, Burgh Daly MD, Murray JG (1957) Functional and histological studies of the vagus nerve and its branches to the heart, lungs and abdominal viscera in the cat. J Physiol 135:182–205
Ahlman BHJ, Larson GM, Bombeck CT, Nyhus LM (1979) Origin of the adrenergic fibers in the subdiaphragmatic vagus of the dog. Am J Surg 137:116–122
Altschuler SM, Bao X, Bieger D, Hopkins DA, Miselis RR (1989) Viscertopic representation of the upper alimentary tract in the rat: Sensory ganglia and nuclei of the solitary and spinal trigeminal tracts. J Comp Neurol 283:248–268
Asala SA, Bower AJ (1986) An electron microscope study of vagus nerve composition in the ferret. Anat Embryol 176:247–253
Bieger D, Hopkins DA (1987) Viscerotopic representation of the upper alimentary tract in the medulla oblongata in the rat: The nucleus ambiguus. J Comp Neurol 262:546–562
Boekelaar AB (1985) The extrinsic innervation of the stomach and other abdominal organs in the rat. MD Thesis Amsterdam
Carlson N, Berthoud H-R, Powley TL (1989) Viscerotopic organization of abdominal vagal branches as determined by induced motility responses. Soc Neurosci Absts 15:264
Conover WJ, Iman RL (1981) Rank transformations as bridge between parametric and nonparametric statistics. Am Statistician 35:124–129
Dahlqvist A, Carlsoo B, Hellstrom S, Domeij S, Kourtopoulos H (1986) Fiber composition of the recurrent laryngeal nerve after experimental vagotomy and sympathectomy, a qualitative study by light and electron microscopy. Acta Anat 125:114–120
Evans DHL, Murray JG (1954) Histological and functional studies on the fibre composition of the vagus nerve of the rabbit. J Anat 93:9–14
Fahrenkam I, Friede RL (1987) Characteristic variation of relative myelin sheath thickness in 11 nerves of the rat. Anat Embryol 177:115–121
Fercenci DA, Altschuler SM, Miselis RR (1989) Representation of cecum in lateral dorsal motor nucleus and commissural subnucleus of the nucleus tractus solitarius in rat. Soc Neurosci Absts 15:264
Fox EA, Powley TL (1984) Regeneration may mediate the sparing of VMH obesity observed with prior vagotomy. Am J Physiol 247:R308-R317
Fox EA, Powley TL (1985) Longitudinal columnar organization within the dorsal motor represents separate branches of the abdominal vagus. Brain Res 341:269–282
Gabella G, Pease HL (1973) Number of axons in the abdominal vagus of the rat. Brain Res 58:465–469
Griffith CA (1969) Significant functions of the hepatic and celiac vagi. Am J Surg 118:251–259
Kemp DR (1973) A histological and functional study of the gastric mucosal innervation in the dog Part I: The quantification of the fiber content of the normal subdiaphragmatic vagal trunks and their abdominal branches. Aust N Z J Surg 43:288–293
Kitchell RL, Stromberg MW, Davis LH (1977) Comparative study of the dorsal motor nucleus of the vagus nerve. Am J Vet Res 38:37–49
Kohno T, Mori S, Mito M (1987) Cells of origin innervating the liver and their axonal projections with synaptic terminals into the liver parenchyma. Hokkaido J Med Sci 62:933–946
Legros G, Griffith CA (1969) The abdominal vagal system in rats. J Surg Res 9:183–186
Loeweneck H (1969) Vagotomie und Pancreasinnervation. Langenbecks Arch Chir 324:44–59
Lu YL, Sakai H (1984a) Cytoarchitectural study on the dorsal motor nucleus of the rat vagus. Okajimas Folia Anat Jpn 61:221–234
Lu YL, Sakai H (1984b) Localization of the neurons of origin of efferent fibers in the glossopharyngeal, vagus and accessory nerves in the rat by means of retrograde degeneration and horeseradish peroxidase methods. Okajimas Folia Anat. Jpn 61:287–310
Mackay TW, Andrews PLR (1983) A comparative study of the vagal innervation of the stomach in man and the ferret. J Anat 136:449–481
McCrea ED (1924) The abdominal distribution of the vagus. J Anat 59:15–40
McDonald DM (1983) Morphology of the rat carotid sinus nerve. II. Number and size of axons. J Neurocytol 12:373–392
McSwiney BA (1931) Innervation of the stomach. Physiol Rev 11:478–514
Mei N, Condamin M, Boyer A (1980) The composition of the vagus nerve of the cat. Cell Tissue Res 209:423–431
Mitchell GAG (1940) A macroscopic study of the nerve supply of the stomach. J Anat 75:50–63
Mitchell GAG, Warwick R (1955) The dorsal vagal nucleus. Acta Anat 25:371–395
Muryobayashi T, Mori J, Fujiwara M, Shimamoto K (1968) Fluorescence histochemical demonstration of adrenergic nerve fibers in the vagus nerve of cats and dogs. Jpn J Pharmacol 18:285–293
Nogren R, Smith GP (1988) Central distribution of subdiaphragmatic vagal branches in the rat. J Comp Neurol 273:207–223
Payer AF (1979) An ultrastructural study of the schwann cell response to axonal degeneration. J Comp Neurol 183:365–384
Peracchia C, Mittler BS (1972) Fixation by means of glutaraldehyde-hydrogen peroxide reaction products. J Cell Biol 53:234–238
Powley TL, Fox EA, Berthoud H-R (1987) Retrograde tracer technique for assessment of selective and total subdiaphragmatic vagotomies. Am J Physiol 253:R361-R370
Prechtl JC, Powley TL (1985) Organization and distribution of the rat subdiaphragmatic vagus and associated paraganglia. J Comp Neurol 235:182–195
Prechtl JC, Powley TL (1986) A versatile method for analyzing autonomic nerve connectivity. Soc Neurosci Absts 12; 1175
Prechtl JC, Powley TL (1987) A light and electron microscopic examination of the vagal hepatic branch of the rat. Anat Embryol 176:115–126
Prechtl JC, Powley TL (1988) Light and electron microscopic analysis of the accessory celiac branch of the vagus nerve. Soc Neurosci Abstr 14:315
Richter CP, Rice KK (1942) The effect of thiamine hydrochloride on the energy value of dextrose studied in rats by the single food choice method. Am J Physiol 137:573–581
Ruckley CV (1964) A study of the variations of the abdominal vagi. Br J Surg 51:569–573
Sawchenko PE, Gold RM (1981) Effects of gastric vs complete subdiaphragmatic vagotomy on hypothalamic hyperphagia and obesity. Physiol Behav 26:281–292
Schwaber JS, Cohen DH (1978) Electrophysiological and electron microscopic analysis of the vagus nerve of the pigeon with particular reference to the cardiac innervation. Brain Res 147:65–78
Sunderland S (1980) The anatomical basis of nerve repair. In: Jewett DL, McCarroll HR (eds) Nerve repair and regeneration: Its clinical and experimental basis, CV Mosby Co, St. Louis Toronto London
Tohyama K, Ide C, Nitatori T, Yokota R (1983) Nearest-neighbor distance of intermediate filaments in axons and Schwann cells. Acta Neuropathol 60:194–198
Winer BJ (1971) Statistical principles in experimental design, 2nd edn. McGraw-Hill, New York
Wood JD (1987) Physiology of the enteric nervous system. In: Johnson LR, Christensen J, Jackson MD, Jacobson ED, Walsh JH (eds) Physiology of the Gastrointestinal Tract, vol 1. Raven Press, New York
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Prechtl, J.C., Powley, T.L. The fiber composition of the abdominal vagus of the rat. Anat Embryol 181, 101–115 (1990). https://doi.org/10.1007/BF00198950
Accepted:
Issue Date:
DOI: https://doi.org/10.1007/BF00198950