Summary
The pharmacokinetics of quinine has been studied in ten healthy adult Africans after intravenous infusion and oral ingestion of a 500 mg dose. Blood and saliva samples were collected over 48 h and quinine in plasma, red cells and saliva was determined by HPLC.
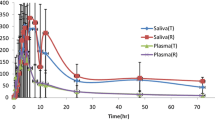
Quinine was rapidly and almost completely absorbed after an oral dose, with absorption half-life of 0.53 h, a tmax of 1–3 h and a bioavailability of 88%. Analysis of the i. v. data gave an apparent volume of distribution of 3.6 1·kg−1 and a plasma clearance of 0.19 l·kg−1·h−1. The concentration-time curves for plasma, red cells and saliva had declining phases were approximately parallel, giving a similar half-life that in all three media. The half-lives after the i. v. infusion also did not different from those after oral administration. The dose was well tolerated by both methods of administration.
Similar content being viewed by others
References
Berlin CM, Stackman JM, Vessel ES (1975) Quinine-induced alteration in drug disposition. Clin Pharmacol Ther 18: 670–679
Ekanem OJ (1985) Plasmodium falciparum falciparum malaria not responding to chloroquine in Nigeria. Trans Roy Soc Trop Med Hyg 79: 141
Jamaludin A, Mohamad M, Navaratnam V, Selliah K, Tan SC, Wernsdorfer WH, Yuen KH (1988) Relative bioavailability of the hydrochloride, sulphate and ethyl carbonate salts of quinine. Br J Clin Pharmacol 25: 261–263
Mansor SM, Taylor TE, McGrath CS, Edwards G, Ward SA, Wirima JJ, Molyneux ME (1990) The safety and kinetics of intramuscular quinine in Malawian children with moderately severe falciparum malaria. Trans Roy Soc Trop Med Hyg 84: 482–487
Matin SB, Wan SH, Karam JH (1974) Pharmacokinetics of tolbutamide: prediction by concentration in saliva. Clin Pharmacol Ther 16: 1052–1058
Mihaly GW, Ching MS, Klejn MB, Paull J, Smallwood RA (1987) Differences in protein binding of quinine and quinidine to plasma proteins. Br J Clin Pharmacol 24: 769–774
Salako LA, Aderounmu AF (1987) In-vitro chloroquine and mefloquine resistant Plasmodium falciparum in Nigeria. Lancet 2: 572–573
Salako LA (1987) Quinine and malaria: The African experience. Acta Leidensia 55: 167–180
White NJ, Looareesuwan S, Warrell DA, Warrell MJ, Bunnag D, Harinasuta T (1982) Quinine pharmacokinetics and toxicity in cerebral and uncomplicated falciparum malaria. Am J Med 73: 564–572
White NJ, Chanthavanich P, Krishna S, Bunch C, Silamut K (1983) Quinine disposition kinetics. Br J Clin Pharmacol 16: 399–404
White NJ (1985) Clinical pharmacokinetics of antimalarial drugs. Clin Pharmacokinet 10: 181–215
WHO Malaria Action Programme (1986) Severe and Complicated Malaria. Trans Roy Soc Trop Med Hyg 80 [Suppl]: 1–50
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Salako, L.A., Sowunmi, A. Disposition of quinine in plasma, red blood cells and saliva after oral and intravenous administration to healthy adult Africans. Eur J Clin Pharmacol 42, 171–174 (1992). https://doi.org/10.1007/BF00278479
Issue Date:
DOI: https://doi.org/10.1007/BF00278479