Abstract
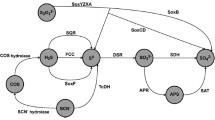
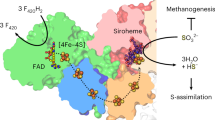
An inducible sulfite reductase was purified from Clostridium pasteurianum. The pH optimum of the enzyme is 7.5 in phosphate buffer. The molecular weight of the reductase was determined to be 83,600 from sodium dodecyl sulfate gel electrophoresis with a proposed molecular structure: α2β2. Its absorption spectrum showed a maximum at 275 nm, a broad shoulder at 370 nm and a very small absorption maximum at 585 nm. No siroheme chromophore was isolated from this reductase. The enzyme could reduced the following substrates in preferential order: NH2OH> SeO 2-3 >NO 2-2 at rates 50% or less of its preferred substrate SO 2-3 . The proposed dissimilatory intermediates, S3O 2-6 or S2O 2-3 , were not utilized by this reductase while KCN inhibited its activity. Varying the substrate concentration [SO 2-3 ] from 1 to 2.5 μmol affected the stoichiometry of the enzyme reaction by alteration of the ratio of H2 uptake to S2- formed from 2.5:1 to 3.1:1. The inducible sulfite reductase was found to be linked to ferredoxin which could be completely replaced by methyl viologen or partially by benzyl viologen. Some of the above-mentioned enzyme properties and physiological considerations indicated that it was a dissimilatory type sulfite reductase.
Similar content being viewed by others
Abbreviations
- SDS:
-
sodium dodecyl sulfate
- BSA:
-
bovine serum albumin
- LDH:
-
Lactate dehydrogenase
References
Akagi JM, Chan M, Adams V (1974) Observations on the bisulfite reductase (P 582) isolated from Desulfotomaculum nigrificans. J Bacteriol 120:240–244
Brewer JM, Pesce AJ, Ashworth RB (1974) Experimental techniques in biochemistry. Prentice-Hall, Inc., Englewood Cliffs, NJ, pp 351–352
Drake HL, Akagi JM (1976) Purification of a unique bisulfite-reducing enzyme from Desulfovibrio vulgaris. Biochem Biophys Res Comm 71:1214–1219
Drake HL, Akagi JM (1977) Bisulfite reductase of Desulfovibrio vulgaris: explanation for product formation. J Bacteriol 32:139–143
Jeng D (1969) Sulfur metabolism of N2-fixing Clostridium pasteurianum. Ph. D. Thesis, Purdue University, West Lafayette, IN, USA
Jones HE, Skyring GW (1975) Effect of enzymatic assay conditions on sulfite reduction catalyzed by desulfoviridin from Desulfovibrio gigas. Biochim Biophys Acta 377:52–60
Hatchikian C, Zeikus JG (1983) Characterization of a new type dissimilatory sulfite reductase present in Thermodesulfo-bacterium commune. J Bacteriol 153:1211–1220
Kobayashi K, Takahashi E, Ishimoto M (1972) Biochemical studies on sulfate-reducing bacteria. XI. Purification and some properties of sulfite reductase, desulfoviridin. J Biochem 72:879–887
Kobayashi K, Seki Y, Ishimoto M (1974) Biochemical studies on sulfate-reducing bacteria. XIII. Sulfite reductase from Desulfovibrio vulgaris — mechanism of trithionate, thiosulfate and sulfide formation and enzymatic properties. J Biochem 75:519–529
Laishley EJ, Krouse HR (1978) Stable isotope fractionation by Clostridium pasteurianum. 2. Regulation of sulfite reductases by sulfur amino acids and their influence on sulfur isotope fractionation during SO 2-3 and SO 2-4 reduction. Can J Microbiol 24:716–724
Laishley EJ, Lin P, Peck Jr HD (1971) A ferredoxin-linked sulfite reductase from Clostridium pasteurianum. Can J Microbiol 17:889–895
Laishley EJ, McCready RGL, Bryant R, Krouse HR (1976) Stable isotope fractionation by C. pasteurianum. In: Nriagu JO (ed) Environmental biochemistry, vol I: Carbon, nitrogen, phosphorus, sulfur and selenium cycles. Ann Arbor Science, Ann Arbor, MI, pp 327–349
Laishley E, Tyler M, Krouse H (1984) Sulfur isotope fractionation during SO 32 reduction by different clostridial species. Can J Microbiol (in press)
Lee PJ, LeGall J, Peck Jr HD (1973) Isolation of assimilatory and dissimilatory-type sulfite reductases from Desulfovibrio vulgaris. J Bacteriol 115:529–542
Mortenson LE (1964) Purification and analysis of ferredoxin from Clostridium pasteurianum. Biochim Biophys Acta 81:71–77
Murphy MJ, Siegel LM (1973) Siroheme and sirohydrochlorin. The basis for a new type of porphyrin-related prosthetic group common to both assimilatory and dissimilatory sulfite reductases. J Biol Chem 248:6911–6919
Palmer WG (1954) Experimental Inorganic Chemistry. Cambridge University Press, New York, pp 370–371
Peck Jr HD (1967) Some evolutionary aspects of inorganic sulfur metabolism. In: Lectures on theoretical and applied aspects of modern microbiology. University of Maryland Press, College Park, MD
Peck Jr HD, Gest H (1956) A new procedure for assay of bacterial hydrogenase. J Bacteriol 71:70–80
Schedel M, Trüper HG (1979) Purification of Thiobacillus denitrificans siroheme sulfite reductase and investigation of some molecular and catalytic properties. Biochim Biophys Acta 568:454–467
Schedel M, Vanselow M, Trüper HG (1979) Siroheme sulfite reductase isolated from Chromatium vinosum: Purification and investigation of some of its molecular and catalytic properties. Arch Microbiol 121:29–36
Schwartz RM, Dayhoff MO (1978) Origins of prokaryotes, cukaryotes, mitochondria and chloroplasts. Science 199: 395–403
Siegel LM, Murphy MJ, Kamin H (1973) Reduced nicotinamide adenine dinucleotide phosphate-sulfite reductase of Enterobacteria. 1. The Escherichia coli hemoflavoprotein: molecular parameters and prosthetic groups. J Biol Chem 248:251–264
Weber K, Osborn M (1969) The reliability of molecular weight determinations by dodecyl sulfate-polyacrylamide gel electrophoresis. J Biol Chem 244:4406–4412
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Harrison, G., Curle, C. & Laishley, E.J. Purification and characterization of an inducible dissimilatory type sulfite reductase from Clostridium pasteurianum . Arch. Microbiol. 138, 72–78 (1984). https://doi.org/10.1007/BF00425411
Received:
Accepted:
Issue Date:
DOI: https://doi.org/10.1007/BF00425411