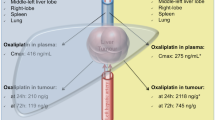
Summary
Fotemustine is a new nitrosourea derivative that contains an alpha-aminophosphonic acid and has a short half-life and a high plasma clearance. As myelosuppression occurs as the dose-limiting toxicity, local drug delivery has been investigated in the treatment of liver metastases arising from colorectal cancer. A pharmacokinetic study was undertaken in patients who received either i. v. or hepatic intra-arterial (HIA) infusion of 100 mg/m2 fotemustine so as to estimate the advantage of local chemotherapy, considering the pharmacokinetic differences between the two routes together with the resultant toxicities (when available). Our findings substantiated the hypothesis that a 4-h HIA infusion of fotemustine would result in a lower exposure of healthy tissues to the drug, since the AUC measured in systemic plasma was reduced by approximately 50% following such treatment as compared with i. v. infusion. This reduction in AUC should indicate a manyfold increase in exposure of the liver tumour to the alkylating properties of the drug, since it represents the proportion of the dose that has degraded within the liver. The first-pass liver-extraction ratio of fotemustine given as a 4-h HIA infusion, which ranged from 0.4 to 0.9 as estimated in patients receiving i. v. and HIA infusions in a cross-over study, argues for further investigation of HIA fotemustine infusion for the treatment of liver metastases so as to increase the response rate and decrease the occurrence of major toxic side effects in such patients.
Similar content being viewed by others
References
Ackerman NB (1986) Vascular patterns of liver tumors and their consequences for different therapeutic approaches. In: Recent results in cancer research — therapeutic strategies in primary and metastatic liver cancer, vol 100. Springer, Berlin Heidelberg New York, pp 248–250
Bleiberg H, Bequart D, Van Oosterom A, Sessa C, Cavalli F, Wils J, Michel J, Lucas C, Gordon B, Campbell D, Gerard B (1989) A clinical and pharmacokinetic study of fotemustine in advanced colorectal cancer. proceedings, ECCO 5, Londres, 3–7 September
Brown CE, Warren S (1983) Visceral metastases from rectal carcinoma. Surg Gynecol Obstet 66: 611–621
Chen HSG, Gross JF (1980) Intra-arterial infusion of anticancer drugs: theoretic aspects of drug delivery and rview of responses. Cancer Treat Rep 64: 31–40
Daly JM, Kemeny N, Botet JH (1984) Long term hepatic arterial chemotherapy. Arch Surg 119 (8): 936–941
Ensminger WD, Gyves JW (1983) Clinical pharmacology of hepatic arterial chemotherapy. Semin Oncol 10: 176–182
Frei E III (1973) Effect of dose and schedule on response In: Holland JF, Frei E III (eds) Cancer medicine. Lea and Febiger, Philadelphia, pp 717–730
Gomeni R (1984) Pharm: An interactive graphic program for individual and population pharmacokinetics parameter estimation. Comput Biol Med 14: 25–34
Gordon BH, Richards RP, Hiley MP, Gray AJ, Ings RMJ, Campbell DB (1989) A new method for the measurement of nitrosoureas in plasma: an HPLC procedure for the measurement of fotemustine kinetics. Xenobiotica 19: 329–339
Ings RMJ, Gray AJ, Taylor AR, Gordon BH, Breen M, Hiley MP, Brownsill R, Marchant N, Richards D, Wallace D, Hughes T, thomas R, Williams J, Lucas C, Campbell DB (1990) Disposition, pharmacokinetics, and metabolism of14C-fotemustine in cancer patients. Eur J Cancer 26: 838–842
Jacquillat C, Khayat D, Banzet P, Weil M, Fumoleau P, Avril MF, Namer M, Bonneterre J, Kerbrat P, Bonerandi JJ, Bugat R, Montcuquet P, Cupissol D, Lanvin R, Vilmer C, Prache C, Bizzari JP (1990) Final report of the French multicentre phase II study of nitrosourea fotemustine in 153 evaluable patients with disseminated malignant melanoma including patients with cerebral metastases. Cancer 66: 1873–1878
Kaplan WD, D'Orsi CJ, Ensminger WD, Smith EH, Levin DC (1978) Intra-arterial radionuclide infusion: a new technique to assess chemotherapy repusion patterns. Cancer Treat Rep 62: 699–703
Kemeny N, Golbey R (1980) A chemotherapeutic approach to colorectal carcinoma. In: Stearns MR Jr (ed) Neoplasma of the colon rectum and anus. John Wiley & Sons, New York, pp 155–168
Kemeny N, Daly J, Oderman P, Shike M, Chun H, Petroni G, Geller N (1984) Hepatic artery pump infusion: toxicity and results in patients with metastatic colorectal carcinoma. J Clin Oncol 2: 595–600
Khayat D, Cour V, Aigner C, Vignoud J, Audhuy B, Monnier A, Lerol A, Bouillet T, Dumesnil Y, Cohen-Aloro G, Bizzari JP, Weil M, Jacquillat C (1990) A phase II study of hepatic intraarterial fotemustine: final report among 66 evaluable patients. J Cancer Res Clin Oncol 116: 692
Khayat D, Cour V, Bizzari JP, Aigner C, Borel C, Cohen-Aloro G Weil M, Auclerc G, Buthiau D, Bousquet JC, Audhuy B, Jacquillat C (1991) Fotemustine in the intraarterial treatment of liver metastasis from malignant melanoma: a phase II study. Am J Clin Oncol 14: 400–404
Lemoine A, Lucas C, Ings RMJ (1991) Metabolism of the chloroethylnitrosoureas. Xenobiotica 21: 775–791
Lokiec F, Bizzari JP, Le Chevalier T, Spielman M, Meeus L, Rouesse J (1987) Influence of infusion time on toxicity and pharmacokinetic parameters of fotemustine. Proceedings, ECCO 4, Madrid, 1–4 November
Lucas C, Ings RMJ, Gray AJ, Deloffre P, Lokiec F, Campbell DB, Beerblock K (1989) Comparaison inter-espèces des paramètres pharmacocinétiques de la fotémustine (nitrosourée S 10036): souris, rat, singe, chien et homme. Bull Cancer (Paris) 76: 863–865
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Fety, R., Lucas, C., Solere, P. et al. Hepatic intra-arterial infusion of fotemustine: pharmacokinetics. Cancer Chemother. Pharmacol. 31, 118–122 (1992). https://doi.org/10.1007/BF00685097
Received:
Accepted:
Issue Date:
DOI: https://doi.org/10.1007/BF00685097