Abstract
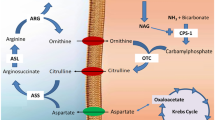
Activity levels of pyruvate dehydrogenase, enzymes of citric acid cycle, aspartate and alanine aminotransferases were estimated in mitochondria, synaptosomes and cytosol isolated from brains of normal rats and those injected with acute and subacute doses of ammonium acetate. In mitochondria isolated from animals treated with acute dose of ammonium acetate, there was an elevation in the activities of pyruvate, isocitrate and succinate dehydrogenases while the activities of malate dehydrogenase (malate→oxaloacetate), aspartate and alanine aminotransferases were suppressed. In subacute conditions a similar profile of change was noticed excepting that there was an elevation in the activity of α-ketoglutarate dehydrogenase in mitochondria. In the synaptosomes isolated from animals administered with acute dose of ammonium acetate, there was an increase in the activities of pyruvate, isocitrate, α-ketoglutarate and succinate dehydrogenases while the changes in the activities of malate dehydrogenase, asparatate and alanine amino transferases were suppressed. In the subacute toxicity similar changes were observed in this fraction except that the activity of malate dehydrogenase (oxaloacetate→malate) was enhanced. In the cytosol, pyruvate dehydrogenase and other enzymes of citric acid cycle except malate dehydrogenase were enhanced in both acute and subacute ammonia toxicity though their activities are lesser than that of mitochondria. In this fraction malate dehydrogenase (oxaloacetate→malate), was enhanced while activities of malate dehydrogenase (malate→oxaloacetate), aspartate, and alanine aminotransferases were suppressed in both the conditions. Based on these results it is concluded that the decreased activities of malate dehydrogenase (malate→oxaloacetate) in mitochondria and of aspartate, aminotransferase in mitochondria and cytosol may be responsible for the disruption of malate-aspartate, shuttle in hyperammonemic state. Possible existence of a small vulnerable population of mitochondria in brain which might degenerate and liberate their contents into cytosol in hyperammonemic states is also suggested.
Similar content being viewed by others
References
Bessman, S. P., and Bessman, A. N. 1955. The cerebral and peripheral uptake of ammonia in liver disease with an hypothesis for the mechanism of hepatic coma. J. Clin. Invest 34:622–628.
Bessman, S. P., and Pal, N. 1976. The Krebs cycle depletion theory of hepatic coma. pp. 83–89, In, Grisolia, S., Baguena, R., and Mayer, F. (eds.) Urea Cycle, John Wiley and sons, New York.
McCandless, D. W., and Schenker, S. 1981. Effect of acute ammonia intoxication of energy stores in the cerebral reticular activating system. Exp. Brain Res. 44:325–330.
Hindfelt, B. 1983. Ammonia intoxication and brain energy metabolism. pp. 474–484, In, Kleinberger G., and Deulsch, E., (eds.), New Aspects of Clinical, Nutrition, Karger, Basel.
Hindfelt, B., Plum, F., and Duffy, T. E. 1977. Effect of acute ammonia intoxication on cerebral metabolism in rats with portacaval shunts. J. Clin. Invest., 59:386–396.
Hindfelt, B., and Siesjo, B. K. 1971. Cerebral effects of acute ammonia intoxication. 1. The influence on extracellular acid-base parameters. Scand. J. Clin. Lab. Invest. 28:353–364.
Hindfelt, B., and Siesjo, B. K. 1971. Cerebral effects of acute ammonia intoxication. II. The effect upon energy metabolism. Scand. J. Clin. Lab. Invest. 28:365–374.
Hindfelt, B. 1975. On mechanisms in hyperammonemic coma with particular reference to hepatic encephalopathy. Ann. New York Acad. Sci. 252:116–123.
Hindfelt, B., and Siesjo, B. K. 1970. The effect of ammonia on the energy metabolism of the rat brain. Life Sci. 9:1021–1028.
Ratnakumari, L., Subbalakshmi, G. Y. C. V., and Murthy, Ch. R. K. 1986. Acute effects of ammonia on the enzymes of citric acid cycle in rat brain. Neurochem Int. 8:115–120.
Cotman, C. W. 1974. Isolation of synaptosomal and synaptic plasma membrane fractions. pp. 445–452,in, Fleischer, S. and Packer, L. (eds.), Methods in enzymology, Vol. XXXI, Academic Press, New York.
Subbalakshmi, G. Y. C. V., and Murthy, Ch. R. K. 1985. Isolation of astrocytes, Neurons and Synaptosomes of rat brain cortex: Distribution of enzymes of glutamate metabolism. Neurochem. Res. 10:239–250.
Lowry, O. H., Rosenbrough, N. J., Farr, A. L., and Randall, R. J. 1951. Protein measurement with the folin phenol reagent. J. Biol. Chem. 193:265–275.
Yoshida, A. 1969. L-Malate dehydrogenase fromBacillus subtilus. pp. 141–142, In, Lowenstein, J. M. (ed.), Methods in enzymology, Vol. XIII, Academic Press, New York.
Bergmeyer H. U., and Bernt, E. 1974. Glutamate oxaloacetate transaminase, Glutamate pyruvate transaminase. pp. 574–579, 727–733, In, Bergmeyer, H. U. (ed.), Methods of enzymatic analysis, Vol. II, Academic press, New York.
Nandakumar, N. V., Murthy, Ch. R. K., Vijayakumari, D., and Swami, K. S. 1973. Axonal protein charges and Succinate dehydrogenase activity in Sheep medulla oblongata. Ind. J. Exptl. Biol. 11:525–528.
Subbalakshmi, G. Y. C. V., and Murthy, Ch. R. K. 1985. Differential response of enzymes of glutamate metabolism in neuronal perikarya and synaptosomes in acute hyperammonemia in rat. Nuerosci. Lett. 59:121–126.
Hawkins, R. A., Miller, A. L., Nielsen, R. C., and Veech, R. L. 1973. The acute action of ammonia on rat brain metabolism in vivo. Biochem. J. 134:1001–1008.
Randle, P. J. 1983. Mitochondrial 2-oxo acid dehydrogenase complexes of animal tissues. Philos. Trans. R. Soc. London. Ser. B, 302:47–57.
Laporte, D. C., and Koshland, Jr., D. E. 1983. Phosphorylation of isocitrate dehydrogenase as a demonstration of enhanced sensitivity in covalent regulation. Nature. 305:286–290.
O'Conner, J. E., Guerri, C., and Grisolia, S. 1984. Effects of ammonia on synaptosomal membranes. Biochem. Biophys. Res. Commun. 119:516–523.
Murthy, Ch. R. K., and Hertz L. Regulation of pyruvate decarboxylation in astrocytes and in neurons in primary cultures in the presence and absence of ammonia. Neurochem. Res. 13:57–61.
Jessy, J., and Murthy, Ch. R. K. 1985. Elevation of transamination of branched chain amino acids in brain in acute ammonia toxicity. Neurochem. Int. 7:1027–1031.
Berl, S. 1971. Cerebral amino acid metabolism in hepatic coma. pp. 71–89, In, Polli, E. E. (ed.), Neurochemistry of Hepatic Coma, Vol. 4, Krager, Basel
Drewis, L. R., and Leino R. L. 1980. Neuron-specific mitochondrial degeneration induced by hyperammonemic and octanoic acidemia. Brain Res. 340:211–218.
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Ratnakumari, L., Murthy, C.R.K. Activities of pyruvate dehydrogenase, enzymes of citric acid cycle, and aminotransferases in the subcellular fractions of cerebral cortex in normal and hyperammonemic rats. Neurochem Res 14, 221–228 (1989). https://doi.org/10.1007/BF00971314
Accepted:
Issue Date:
DOI: https://doi.org/10.1007/BF00971314