Abstract
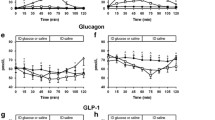
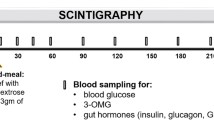
Postprandial glucagon-like peptide-1 (GLP-1), pancreatic glucagon, and insulin were measured in 27 tumor-free patients 43 months (median) after total gastrectomy and in four controls using a99technetium-labeled 100-g carbohydrate solid test meal. Emptying of the gastric substitute was measured by scintigraphy. Fourteen patients suffered from early dumping symptoms, and five of them also reported symptoms suggestive of reactive hypoglycemia (late dumping). The median emptying half-time (T1/2) of the gastric substitute was 480 sec. Sigstad's dumping score was 8.5±1.6 (mean±se) in patients with rapid emptying (T1/2<480 sec), and 3.0±1.5 in patients with slow emptying of the gastric substitute (P=0.02). The peak postprandial concentration of GLP-1 was 44±20 pmol/liter in controls, 172±50 in patients without reactive hypoglycemia, and 502±116 in patients whose glucose fell below 3.8 mmol/liter during the second postprandial hour. Plasma GLP-1 concentrations peaked at 15 min, and insulin concentrations at 30 min after the end of the meal. A close correlation between integrated GLP-1 responses and integrated insulin responses (r=0.68) was observed. Multiple regression revealed that three factors were significantly associated with the integrated glucose concentrations during the second hour (60–120 min): Early (first 30 min) integrated GLP-1 (inverse correlation;P=0.006), age (P=0.006), and early integrated pancreatic glucagon (P=0.005). There was a close (inverse) relationship ofT1/2 with early integrated GLP-1 and pancreatic glucagon, but not with insulin. Gel filtration of pooled postprandial plasma of gastrectomized individuals revealed that all glucagon-like immunoreactivity eluted atK d 0.30 (K d , coefficient of distribution), the elution position of glicentin. Almost all of the GLP-1 like immunoreactivity eluted atK d 0.60, the elution position of gut GLP-1. The authors contend that GLP-1-induced insulin release and inhibition of pancreatic glucagon both contribute to the reactive hypoglycemia encountered in some patients following gastric surgery. Rapid emptying seems to be one causative factor for the exaggerated GLP-1 release in these subjects.
Similar content being viewed by others
References
Wapnick S, Jones JJ: Changes in glucose tolerance and serum insulin following partial gastrectomy and intestinal resection. Gut 13:871–873, 1972
Lauritsen KB, Frederiksen HJ, Uhrenholdt A, Holst JJ: The correlation between gastric emptying time and the response of GIP and enteroglucagon to oral glucose in duodenal ulcer patients. Scand J Gastroenterol 17:513–516, 1982
Sagor GR, Ghatei MA, McGregor GP, Mitchenere P, Kirk RM, Bloom SR: The influence of an intact pylorus on postprandial enteroglucagon and neurotensin release after upper gastric surgery. Br J Surg 68:190–193, 1981
Jenkins DJA, Bloom SR, Albuquerque RH, Leeds AR, Sarson DL, Metz GL, Alberti KGMM: Pectin and complications after gastric surgery: normalisation of postprandial glucose and endocrine responses. Gut 21:574–579, 1980
Bloom SR, Royston CMS, Thomson JPS: Enteroglucagon release in the dumping syndrome. Lancet 2:788–791, 1972
Thomson JPS, Bloom SR: Plasma enteroglucagon and plasma volume change after gastric surgery. Clin Sci Mol Med 51:177–183, 1976
Lawaetz O, Blackburn AM, Bloom SR, Aritas Y, Ralphs DNL: Gut hormone profile and gastric emptying in the dumping syndrome. Scand J Gastroenterol 18:73–80, 1983
Bell GI, Sanchez-Pescador R, Laybourn PJ, Najarian RC: Exon duplication and divergence in the human preproglucagon gene. Nature 304:369–371, 1983
Heinrich G, Gros P, Habener JF: Glucagon gene sequence four of six exons encode separate functional domains of rat preproglucagon. J Biol Chem 259:14082–14088, 1984
Ørskov C, Holst JJ, Knuhtsen S, Baldissera FGA, Poulsen SS, Nielsen OV: Glucagon-like peptides GLP-1 and GLP-2, predicted products of the glucagon gene, are secreted separately from pig small intestine but not pancreas. Endocrinology 119:1467–1475, 1986
Holst JJ, Ørskov C, Nielsen OV, Schwartz TW: Truncated glucagonlike peptide-1, an insulin releasing hormone from the distal gut. FEBS Lett 211:169–174, 1987
Mojsov S, Weir GC, Habener JF: Insulinotropin: Glucagon-like peptide-1 (7–37) encoded in the glucagon gene is a potent stimulator of insulin release in the perfused rat pancreas. J Clin Invest 79:616–619, 1987
Kreymann B, Ghatei MA, Williams G, Bloom SR: Glucagon-like peptide-1 (7–36): A physiological incretin in man. Lancet 2:1300–1305, 1987
Sigstad H: A clinical diagnostic index in the diagnosis of the dumpings syndrome. Acta Med Scand 188:479–486, 1970
Miholic J, Meyer HJ, Kotzerke J, Balks HJ, Aebert H, Jähne J, Pichlmayr R: Emptying of the gastric substitute after total gastrectomy: Roux-y esophagojejunostomy vs jejunal interposition. Ann Surg 210:39–46, 1989
Ørskov C, Holst JJ: Radio-immunoassays for glucagon-like peptides 1 and 2 (GLP-1 and GLP-2). Scand J Clin Lab Invest 47:165–174, 1987
Ørskov C, Holst JJ, Poulsen SS, Kirkegaard P: Pancreatic and intestinal processing of proglucagon in man. Diabetologia 30:874–881, 1987
Holst JJ: Evidence that peak II GLI or enteroglucagon is identical to the C-terminal sequence (residues 33–69) of glicentin. Biochem J 207:381–388, 1982
Bradley EL, Isaacs J, Harsh T, Davidson ED, Millikan W: Nutritional consequences of total gastrectomy. Ann Surg 182:415–429, 1975
Huguier M, Lancret JM, Bernard PF, Baschet C, LeHenand F: Functional results of different reconstructive procedures after total gastrectomy. Br J Surg 63:704–708, 1976
Miholic J, Meyer HJ, Müller MJ, Weimann A, Pichlmayr R: Nutritional consequences of total gastrectomy: the relationship between mode of reconstruction, postprandial symptoms and body composition. Surgery 108:488–494, 1990
Hertz AF: The cause and treatment of some unfavorable after-effects of gastroenterostomy. Ann Surg 58:466–472, 1913
Hobsley M: Dumping and diarrhoea. Br J Surg 68:681–684, 1981
Roberts KE, Randall HT, Farr HW, Kidwell APP, McNeer GP, Pack GT: Cardiovascular and blood volume alterations resulting from intrajejunal administration of hypertonic solutions to gastrectomized patients: The relationship of these changes to the dumping syndrome. Ann Surg 140:631–640, 1954
Peddie GH, Jordan GL, DeBakey ME: Further studies on the pathogenesis of the postgastrectomy syndrome. Ann Surg 146:892–898, 1957
Holst JJ: Enteroglucagon. Adv Meta Disorders 11:393–419, 1988
Thim L, Moody AJ: The primary structure of porcine glicentin (proglucagon). Regul Pept 2:139–151, 1981
Baldissera FGA, Holst JJ: Glucagon-related peptides in the human gastrointestinal mucosa. Diabetologia 26:223–228, 1985
Thim L, Moody AJ: Purification and biochemical characterization of glicentin-related pancreatic peptide (proglucagon fragment) from porcine pancreas. Biochim Biophys Acta 703:134–141, 1982
Ørskov C, Bersani M, Johnsen AH, Højrup P, Holst JJ: Complete sequences of glucagon-like peptide-1 from human and pig small intestine. J Biol Chem 264:12826–12829, 1989
Buhl T, Thim L, Kofod H, Ørskov C, Harling H, Holst JJ: Naturally occurring products of proglucagon 111–160 in the porcine and human small intestine. J Biol Chem 263:8621–8624, 1988
Ørskov C, Buhl T, Rabenhøj L, Lofod H, Holst JJ: Carboxypeptidase-B like processing of the C-terminus of glucagon-like peptide-2 in pig and human small intestine. FEBS Lett 247:193–196, 1989
Schjoldager BTG, Baldissera FGA, Mortensen PE, Holst JJ, Christiansen J: Oxyntomodulin: A potential hormone from the distal gut. Pharmacokinetics and effects on gastric acid and insulin secretion in man. Eur J Clin Invest 18:499–503, 1988
Jarrousse C, Bataille D, Jeanrenaud B: A pure enteroglucagon, oxyntomodulin (glucagon-37), stimulates insulin release in perfused rat pancreas. Endocrinology 115:102–105, 1984
Holst JJ: Gut glucagon, enteroglucagon, gut GLI, glicentin-current status. Gastroenterology 84:1602–1613, 1983
Marco J, Baroja IM, Diaz-Fierros M, Villanueva ML, Valverde I: Relationship between insulin and gut glucagon-like immunoreactivity (GLI) secretion in normal and gastrectomized subjects. J Clin Endocrinol 34:188–191, 1972
Shima K, Kuruda K, Matsuyama T, Tarui S, Nishikawa M: Plasma glucagon and insulin responses to various sugars in gastrectomized and normal subjects. Proc Soc Exper Bio Med 139:1042–1048, 1971
Rehfeld JF, Heding LG, Holst JJ: Increased gut glucagon release as pathogenic factor in reactive hypoglycemia? Lancet 1:116–118, 1973
Shima S, Tabata M, Tanaka A, Kodaira T, Nishino T, Kumahara Y: Exaggerated response of plasma glucagon-like immunoreactivity (GLI) to oral glucose in patients with reactive hypoglycemia. Endocrinol Jpn 28:249–256, 1981
Holst JJ, Sørensen TIA, Nyboe Anderson A, Andersen B, Stadil F, Lauritsen KB, Klein HC: Plasma enteroglucagon after jejuno-ileal bypass with 3 to 1 and 1 to 3 jejunoileal ratio. Scand J Gastroenterol 14:205–207, 1979
Lauritsen KB, Moody AJ, Christensen KC, Jensen SL: Gastric inhibitory polypeptide (GIP) and insulin release after small-bowel resection in man. Scand J Gastroenterol 15:833–840, 1980
Holst JJ: Pattern of glucagon release.In Gut Hormones. SR Bloom (ed). London, Churchill-Livingstone, 1981, pp 325–338
Creutzfeldt W, Ebert R: New developments in the incretin concept. Diabetologie 28:565–573, 1985
Nauck M, Schmidt WE, Ebert R, Strietzel J, Cantor P, Hoffmann G, Creutzfeldt W: Insulinotropic properties of synthetic human gastric inhibitory polypeptide in man: Interactions with glucose, phenylalanine, and cholecystokinin-8. J Clin Endo Metab 69:654–661, 1989
Schattenmann G, Ebert R, Siewert R, Creutzfeldt W: Different response of gastric inhibitory polypeptide to glucose and fat from duodenum and jejunum. Scand J Gastroenterol 19:260–266, 1984
Ørskov C, Holst JJ, Nielsen OV: Effect of truncated glucagon-like peptide-1 (proglucagon 78–107 amide) on endocrine secretion of pig pancreas, antrum, and nonantral stomach. Endocrinology 123:2009–2013, 1988
Elahi D, Meneilly GS, Sclater A, Wong G, McAloon-Dyke, Mojsov S, Habener J, Minaker K, Andersen DK: The insulinotropic effect of GIP: A dose response comparison to glucagonlike peptide-1 (7–37) amine (GLP). Digestion 46:(S1):28, 1990
Forbes GB: Human Body Composition. Growth, Aging, Nutrition and Activity. Springer-Verlag, New York, 1987, pp 178–193
Cherrington AD, Liljenquist JE: Role of glucagon in regulating glucose production in vivo.In Glucagon Physiology, Pathophysiology and Morphology of the Pancreatic A-Cells. RH Unger, L Orci (eds). Amsterdam, Elsevier, 1981, pp 221–254
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Miholic, J., Ørskov, C., Holst, J.J. et al. Emptying of the gastric substitute, glucagon-like peptide-1 (GLP-1), and reactive hypoglycemia after total gastrectomy. Digest Dis Sci 36, 1361–1370 (1991). https://doi.org/10.1007/BF01296800
Received:
Revised:
Accepted:
Issue Date:
DOI: https://doi.org/10.1007/BF01296800