Abstract
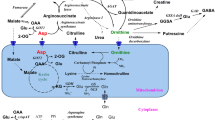
The dibasic amino acids arginine (ARG), ornithine (ORN) and lysine (LYS) are transported by a common saturable transporter (system γ+) at the blood-brain barrier (BBB). In the present study we compared the brain uptake index (BUI) for radiolabelled ORN, ARG and LYS in control rats and in rats treated with thioacetamide (TAA) to induce hepatic encephalopathy (HE). Some animals received i.v. ornithine aspartate (OA), a drug structurally related to the γ+ substrates that ameliorates neurological symptoms following liver damage by improving detoxification of ammonia in peripheral tissues: the compound was administered either by continuous infusion for 6h at a concentration of 2 g/kg (final blood concentration ranging from 0.19–0.5 mM), or as a 15 sec. bolus together with the radiolabelled amino acids, at a concentration of 0.35 mM. TAA treatment resulted in a delayed and progressive increase of BUI for ORN, to 186% of control at 7d post-treatment and to 345% of control at 21d post-treatment, when despite sustained liver damage, HE symptoms were already absent. In contrast, the BUI for ARG decreased to 30% of control at 7d post-treatment and remained low (42% of control) at 21d post-treatment. A 6h infusion of OA to untreated rats resulted in a reduction of the BUI for ARG and ORN to 51% and 62% of the control levels, respectively. Reductions of a similar magnitude were noted with both amino acids following the 15 sec OA bolus, indicating direct interaction of OA with the transport site in both cases. OA administered by either route abolished the enhancement of BUI for ORN, but did not further inhibit the BUI for ARG in the TAA-treated animals. The results indicate that some as yet unspecified factors released from damaged liver either modify the structure or conformation of the γ+ transporter at the BBB from the normally ARG-preferring to the ORN-preferring state, or activate (induce) a different transporter specific for ORN which is normally latent.
Similar content being viewed by others
References
Albrecht, J., and Hilgier, W. (1984). Brain carbonic anhydrase activity in rats in experimental hepatic encephalopathy.Neurosci. Lett. 45:7–10.
Albrecht, J., and Hilgier, W. (1986). Arginine in thioacetamide-induced hepatic encephalopathy in rats: activation of enzymes of arginine metabolism to glutamate.Acta Neurol. Scand. 73:498–501.
Albrecht J., Hilgier, W., Lazarewicz, J.W., Rafalowska, U., and Wysmyk-Cybula, U. (1988). Astrocytes in acute hepatic encephalopathy: Metabolic properties and transport functions. In (M.D. Norenberg, L. Hertz, A. Schousboe A., eds.)Biochemical Pathology of Astrocytes New York, Alan R. Liss, Inc., pp. 465–476.
Albrecht, J., Hilgier, W., and U. Rafalowska, U. (1990). Activation of arginine metabolism to glutamate in rat brain in thioacetamide-induced hepatic encephalopathy: an adaptive response?,J. Neurosci. Res. 25:125–130.
Albrecht, J., Hilgier, W., Januszewski, S., Kapuscinski, A., and Quack, G. (1994). Increase in the brain uptake index for L-ornithine in rats with hepatic encephalopathy.NeuroReport,5:671–673.
Aoki E., Semba, R., Mikoshiba, K. and Kashiwamata, S. (1991). Predominant localization in glial cells of free L-arginine. Immunocytochemical evidence.Brain Res. 547:190–192.
Banos, G., Daniel, P.M., and Pratt, E.O. (1974). Saturation of a shared mechanism which transports L-arginine and L-lysine into the brain of the living rat.J. Physiol. (Lond.) 236:29–41.
Cangiano, C., Cardelli-Cangiano, P., Cascino, A., Ceci, F., Fiori, A., Mulieri, M. et al. (1988). Uptake of amino acids by brain microvessels isolated from rats with experimental chronic liver failure.J. Neurochem. 51:1675–1681.
Christensen, H.N. (1990). Role of amino acid transport and countertransport in nutrition and metabolism.Physiol. Rev. 70:43–77.
Garthwaite, J. (1991). Glutamate, nitric oxide and cell signalling in the central nervous system.Brain Res. 547:190–192.
Greenstein, J.P., and Winitz M. (1961). Amino acids as dipolar ions. In: (J.P. Greenstein and M. Winitz, eds.)Chemistry of the Amino Acids, Wiley, New York, pp. 455–471.
James, J.H., Escourrou, J., and Fisher, J.E. (1979). Blood-brain neutral amino acid transport activity is increased after portacaval anastomosis.Science 200:1395–1397.
Hilgier, W., Albrecht, J. and Krasnicka, Z. (1983). Thioacetamide-induced hepatic encephalopathy in the rat. I Preliminary morphological and biochemical observations.Neuropatol. Pol. 21:487–494.
Johnson, J.L. and Roberts, E. (1984). Proline, glutamate and glutamine metabolism in mouse brain synaptosomes.Brain Res. 323:247–256.
Klausner R.D., Kleinfeld, A.M., Hoover, R.L., and Karnovsky, M.J. (1980). Lipid domains in membranes. Evidence derived from structural perturbations induced by free fatty acids and life time heterogeneity analysis.J. Biol. Chem. 255:11286–11295.
Kircheis, G., Quack, G. and Erbler, H. (1994). L-ornithine-L-aspartate in the treatment of hepatic encephalopathy. In (H.O. Conn, and J. Bircher, eds.):Hepatic Encephalopathy: Syndromes and Therapies, Medi-Ed Press, Bloomington, Ill., pp. 373–386.
Knudsen, G.M., Pettigrew, K.D., Patlak, C.S., Hertz, M.M., and Paulson, O.B. (1990). Assymetrical transport of amino acids across blood-brain barrier in humans.J. Cerebr. Blood Flow. Met. 10:698–706.
Lopes, M.C., Cardoso, S.A., Schousboe, A., and Carvalho, A.P. (1994). Amino acids differentially inhibit L-[3H]arginine transport and nitric oxide synthase in rat brain synaptosomes.Neurosci. Lett. 181:1–4.
Mans A.M., Biebuyck, J.F., Shelly, K., and Hawkins, R.A. (1982). Regional blood-brain barrier permeability to amino acids after portacaval anastomosis.J. Neurochem. 38:705–717.
McGivan, J.D., and Pastor-Anglada, M. (1994). Regulatory and molecular aspects of mammalian amino acid transport.Biochem. J. 299:321–334.
Metoki, K., and Hommes, F.A. (1984). The uptake of ornithine and lysine by isolated hepatocytes and fibroblasts.Int. J. Biochem. 16:833–836.
Norenberg, M.D. (1986). Hepatic encephalopathy: a disorder of astrocytes. In (S. Fedoroff and A. Vernadakis eds.)Astrocytes, Academic Press, New York, pp. 425–460.
O'Connor, J.R., Guerri, C., and Grisolia, S. (1984). Effects of ammonia on synaptosomal membranes.Biochem. Biophys. Res. Comm. 119:516–523.
Oldendorf, W.H. (1971). Brain uptake of radiolabeled amino acids, amines, and hexoses after arterial injection.Am. J. Physiol. 230:94–98.
Oldendorf, W.H., and Szabo, J. (1976). Amino acid assignment to one of the three blood-brain barrier amino acid carriers.Am. J. Physiol. 230:94–98.
Pow, D.V. (1994). Immunocytochemical evidence for a glial localization of arginine, and neuronal localization of citrulline in the rat neurohypophysis: implications for nitrergic transmission.Neurosci. Lett. 181:141–144.
Sarhan, S., Knoedgen, B., and Seiler, N. (1994). Protection against lethal ammonia intoxication: synergism between endogenous ornithine and 1-carnitine.Metab. Brain Dis. 9:67–79.
Schmidlin, A., and Wiesinger, H. (1994). Transport of L-arginine in cultured glial cells.Glia 11:262–268.
Schmidt, K., Klatt, P., and Mayer, B. (1993). Characterization of endothelial cell amino acid systems involved in the actions of nitric oxide synthase inhibitors.Mol. Pharmacol. 44:615–621.
Shank, R.P., and Campbell, G.L.M. (1993). Ornithine as a precursor of glutamate and GABA: Uptake and metabolism by neuronal and glial enriched cellular material.J. Neurosci. Res. 9:47–57.
Snyder, S.H., and Bredt, D.S. (1991). Nitric oxide as a neuronal messenger.Trends Pharmacol. Sci. 12:125–128.
Steadt, U., Lewelling, H., Gladisch, R., Kortsik, C., Hagmüller, E., and Holm, E. (1993). Effects of ornithine aspartate on plasma ammonia and plasma amino acids in patients with cirrhosis. A double-blind, randomized study using a four-fold crossover design.J. Hepatol. 19:424–430.
Stoll, J., Wadhwani, K.C., and Smith, Q.R. (1993). Identification of the cationic amino acid transporter (system γ+) of the rat blood-brain barrier.J. Neurochem. 60:1956–1959.
Trout, J.J., Koenig, H., Goldstone, A.D., Iqbal, Z., Lu, Ch. Y., and Siddiqui, F. (1993). N-methyl-D-aspartate receptor excitotoxicity involves activation of polyamine synthesis: Protection by α-difluoro- methylornithine.J. Neurochem. 60:352–355.
Westergaard, N., Beart, P.M., and Schousboe, A. (1993). Transport of L-[3H]arginine in cultured neurons: Characteristics and inhibition by nitric oxide synthase inhibitors.J. Neurochem. 61:364–367.
White, M.F. (1985). The transport of cationic amino acids across the plasma membrane of mammalian cells.Biochim. Biophys. Acta 822:355–374.
White, M.F., Gazzola, G.C., and Christensen, H.N. (1982). Cationic amino acid transport into cultured animal cells. I. Influx into cultured human fibroblasts.J. Biol. Chem. 257:4443–4449.
Williams, K.W. (1995). Pharmacological properties of recombinant N-methyl-D-aspartate (NMDA) receptors containing ε4 (NR2D) subunit.Neurosci. Lett. 194:181–184.
Williams, K., Dawson, V.L., Romano, C., Dichter, M.A., and Molinoff, P.B. (1990). Characterization of polyamines having agonist, antagonist, and inverse agonist effects at the polyamine recognition site of the NMDA receptor.Neuron,5:199–208.
Zanchin, G., Rigotti, P., Dussini, N., Vassanelli, P., and Battistin, L. (1979). Cerebral amino acid levels and uptake in rats after portacaval anastomosis: II. Regional studiesin vivo.J. Neurosci Res. 4:301–310.
Zieve, L. (1987). Pathogenesis of hepatic encephalopathy.Metab. Brain Dis. 2:147–165.
Zimmermann, Ch., Ferenci, P., Pifl, Ch., Yurdaydin, C., Ebner, J., Lassmann, H.et al. (1989). Hepatic encephalopathy in thioacetamide-induced liver failure in rats: Characterization of an improved model and study of amino acid-ergic neurotransmission.Hepatology,9:594–601.
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Albrecht, J., Hilgier, W., Januszewski, S. et al. Contrasting effects of thioacetamide-induced liver damage on the brain uptake indices of ornithine, arginine and lysine: Modulation by treatment with ornithine aspartate. Metab Brain Dis 11, 229–237 (1996). https://doi.org/10.1007/BF02237960
Received:
Accepted:
Issue Date:
DOI: https://doi.org/10.1007/BF02237960