Abstract
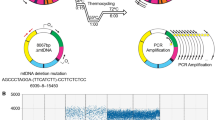
Abundant evidence has been gathered to suggest that mitochondrial DNA (mtDNA) sustains many more mutations and greater oxidative damage than does nuclear DNA in human tissues. Uremic patients are subject to a state of enhanced oxidative stress due to excess production of oxidants and a defective antioxidant defense system. This study was conducted to investigate mtDNA mutations and oxidative damage in skeletal muscle of patients with chronic uremia. Results showed that large-scale deletions between nucleotide position (np) 7,900 and 16,300 of mtDNA occurred at a high frequency in muscle of uremic patients. Among them, the 4,977-bp deletion (mtDNA4977) was the most frequent and most abundant large-scale mtDNA deletion in uremic skeletal muscle. The proportion of mtDNA4977 was found to correlate positively with the level of 8-hydroxy 2′-deoxyguanosine (8-OHdG) in the total DNA of skeletal muscle (r=0.62, p<0.05). Using long-range PCR and DNA sequencing, we identified and characterized multiple deletions of mtDNA in skeletal muscle of 16 of 19 uremic patients examined. The 8,041-bp deletion, which occurred between np 8035 and 16,075, was flanked by a 5-bp direct repeat of 5′-CCCAT-3′. Some of the deletions were found in more than 1 patient. On the other hand, we found that the mean 8-OHdG/105 dG ratio in the total cellular DNA of muscle of uremic patients was significantly higher than that of the controls (182.7 ± 63.6 vs. 50.9 ± 21.5, p=0.05). In addition, the mean 8-OHdG/105 dG ratio in muscle mtDNA of uremic patients was significantly higher than that in nuclear DNA (344.0 ± 56.9 vs. 146.3 ± 95.8, p=0.001). Moreover, we found that the average content of lipid peroxides in mitochondrial membranes of skeletal muscle of uremic patients was significantly higher than that of age-matched healthy subjects (23.76 ± 6.06 vs. 7.67 ± 0.95 nmol/mg protein; p<0.05). The average content of protein carbonyls in the mitochondrial membranes prepared from uremic skeletal muscles was significantly higher than that in normal controls (24.90 ± 4.00 vs. 14.48 ± 1.13 nmol/mg protein; p<0.05). Taken together, these findings suggest that chronic uremia leads to mtDNA mutations together with enhanced oxidative damage to DNA, lipids, and proteins of mitochondria in skeletal muscle, which may contribute to the impairment of mitochondrial bioenergetic function and to skeletal myopathy commonly seen in uremic patients.
Similar content being viewed by others
References
Ames BN, Shigenaga MK, Hagen TM. Oxidants, antioxidants and the degenerative diseases of aging. Proc Natl Acad Sci USA 90:7915–7922;1993.
Anderson S, Bankier AT, Barrell BG, de Brujin MHL, Coulson AR, Drouin J, Eperon IC, Nierlich DP, Roe BA, Sanger F, Schreier PH, Smith AJH, Staden R, Young IG. Sequence and organization of the human mitochondrial genome. Nature 290:457–465;1981.
Canaud B, Cristol JP, Morena M, Leray-Moragues H, Bosc JY, Vaussenat F. Imbalance of oxidants and antioxidants in hemodialysis patients. Blood Purif 17:99–106;1999.
Canestrari F, Galli F, Giorgini A, Albertini MC, Galiotta P, Pascucci M Bossu M. Erythrocyte redox state in uremic anemia: Effects of hemodialysis and relevance of glutathione metabolism. Acta Hematol 91:187–193;1994.
Chen JJ, Yu BP. Alterations in mitochondrial membrane fluidity by lipid peroxidation products. Free Radic Biol Med 17:411–418;1994.
Chen SS, Chang LS, Wei YH. Oxidative damage to proteins and decrease of antioxidant capacity in patients with varicocele. Free Radic Biol Med 30:1328–1334;2001.
Chevion M, Berenshtein E, Stadtman ER. Human studies related to protein oxidation: Protein carbonyl content as a marker of damage. Free Radic Res 33:S99–108;2000.
Davis LJ, Tadolini B, Biagi PL, Walford R, Licastro F. Effect of age and extent of dietary restriction on hepatic microsomal lipid peroxidation potential in mice. Mech Ageing Dev 72:155–163;1993.
Fahal IH, Bell GM, Bone JM, Edwards HT. Physiological abnormalities of skeletal muscle in dialysis patients. Nephrol Dial Transplant 12:119–127;1997.
Garibaldi S, Aragno I, Odetti P, Marinari UM. Relationships between protein carbonyls, retinal and tocopherol level in human plasma. Biochem Mol Biol Int 34:729–736;1994.
Grosovsky AJ, De Boer JG, De Jong PJ, Drobetsky EA, Glickman BW. Base substitutions, frameshifts and small deletions constitute ionizing radiation-induced point mutations in mammalian cells. Proc Natl Acad Sci USA 85:185–188;1988.
Hou JH, Wei YH. The unusual structures of the hot-regions flanking large-scale deletions in human mitochondrial DNA. Biochem J 318:1065–1070;1996.
Ikebe S, Tanaka M, Ohno K, Sato W, Hattori K, Kondo T, Mizuno Y, Ozawa T. Increase of deleted mitochondrial DNA in the striatum in Parkinson's disease and senescence. Biochim Biophys Res Commun 170:1044–1048;1990.
Johns DR, Rutledge SL, Stine OC, Hurko O. Directly repeated sequences associated with pathogenic mitochondrial DNA deletions. Proc Natl Acad Sci USA 86:8059–8062;1989.
Kao SH, Chao HT, Wei YH. Multiple deletions of mitochondrial DNA are associated with the decline of motility and fertility of human spermatozoa. Mol Hum Reprod 4:657–666;1998.
Koenig JS, Fischer M, Bulant E, Tiran B, Elmadfa I, Druml W. Antioxidant status in patients on chronic hemodialysis therapy. Impact of parenteral selenium supplementation. Wien Klin Wochenschr 109:13–19;1997.
Laganiere S, Yu BP. Modulation of membrane phospholipid fatty acid composition by age and food restriction. Gerontology 39:7–18;1993.
Lee HC, Pang CY, Hsu HS, Wei YH. Differential accumulations of 4977 bp deletion in mitochondrial DNA of various tissues in human ageing. Biochim Biophys Acta 1226;37–43;1994.
Lezza AM, Mecocci P, Cormio A, Beal MF, Cherubini A, Cantatore P, Senin U, Gadaleta MN. Mitochondrial DNA 4977 bp deletion and OH8dG levels correlate in the brain of aged subjects but not Alzheimer's disease patients. FASEB J 13:1083–1088;1999.
Lim PS, Cheng YM, Wei YH. Large-scale mitochondrial DNA deletions in skeletal muscle of patients with end-stage renal disease. Free Radic Biol Med 29:454–463;2000.
Lim PS, Wei YH, Yu YL, Kho B. Enhanced oxidative stress in hemodialysis patients receiving intravenous iron therapy. Nephrol Dial Transplant 14:2680–2687;1999.
Lyras L, Cairns N, Jenner A, Jenner P, Halliwell B. An assessment of oxidative damage to proteins, lipids and DNA in brain from patients with Alzheimer's disease. J Neurochem 68:2061–2069;1997.
Maggi E, Bellazzi R, Falaschi F, Frattoni A, Perani G, Finardi G, Gazo A, Nai M, Romanini D, Bellomo G. Enhanced LDL oxidation in uremic patients: An additional mechanism for accelerated atherosclerosis? Kidney Int 45:876–883;1994.
Mecocci P, Fano G, Fulle S, MacGarvey U, Shinobu L, Polidoro MC, Cherubini A, Vechiet J, Senin U, Beal MF. Age-dependent increases in oxidative damage to DNA, lipids and proteins in human skeletal muscle. Free Radic Biol Med 26:303–308;1999.
Mecocci P, MacGarvey U, Kaufman AE, Koontz D, Shoffner JM, Wallace DC, Beal MF. Oxidative damage to mitochondrial DNA shows marked age-dependent increase in human brain. Ann Neurol 34:609–616;1993.
Mita S, Rizzulo R, Moraes CT, Shanske S, Arnaudo E, Fabrizi GM, Koga Y, DiMauro S, Schon EA. Recombination via flanking direct repeats is a major cause of large-scale deletions of human mitochondrial DNA. Nucleic Acids Res 18:561–567;1990.
Miyata T, Fu MX, Kurokawa K, Van Ypersele DE, Strihou C, Thorpe SR, Baynes JW. Products of autooxidation of both carbohydrates and lipids are increased in uremic plasma. Evidence for a generalized increase in oxidative stress in uremia? Kidney Int 54:1290–1295;1998.
Moore GE, Bertocci LA, Painter PL.31P-Magnetic resonance spectroscopy assessment of subnormal oxidative metabolism in skeletal muscle of renal failure patients. J Clin Invest 91:420–424;1993.
Odetti P, Garibaldi S, Gurreri G, Aragno I, Dapino D, Pronzato MA, Marinari UM. Protein oxidation in hemodialysis and kidney transplantation. Metabolism 45:1319–1322;1996.
Ozawa T. Mitochondrial cardiomyopathy. Herz 19:105–118;1994.
Reznick AZ, Packer L. Oxidative damage to proteins: Spectrophotometric method for carbonyl assay. Methods Enzymol 233:357–363;1994.
Richter C. Reactive oxygen and DNA damage in the mitochondria. Mutat Res 275:249–255;1992.
Richter C, Park JW, Ames BN. Normal oxidative damage to mitochondrial and nuclear DNA is extensive. Proc Natl Acad Sci USA 85:6465–6467;1988.
Roselaar SE, Nazhat NB, Winyard PG, Jones P, Cunnigham J, Blake DR. Detection of oxidant in uremic plasma by electron spin resonance spectroscopy. Kidney Int 48:199–206;1995.
Sevanian A: Hochstein P. Mechanisms and consequences of lipid peroxidation in biological systems. Annu Rev Nutr 5:365–390;1985.
Shigenaga MK, Aboujaoude EN, Chen Q, Ames BN. Assays of oxidative DNA damage biomarkers 8-oxo-2′-deoxyguanosine and 8-oxoguanine in nuclear DNA and biological fluids by high performance liquid chromatography with electrochemical detection. Methods Enzymol 234:16–33;1994.
Shigenaga MK, Hagen TM, Ames BN. Oxidative damage and mitochondrial decay in aging. Proc Natl Acad Sci USA 91:10771–10778;1994.
Soong NW, Hinton DR, Cortopassi G, Arnheim N. Mosaicism for a specific somatic mitochondrial DNA mutation in adult human brain. Nat Genet 2:318–323;1992.
Tanaka M, Ozawa T. Analysis of mitochondrial DNA mutations. In: Longstaff A, Revest P (eds): Methods in Molecular Biology. Humana Press, Totowa, pp. 1–28, 1992.
Tarng DC, Huang TP, Wei YH, Liu TY, Chen HW, Chen TW, Yang WC. 8-Hydroxy-2′-deoxyguanosine of leukocyte DNA as a marker of oxidative stress in chronic hemodialysis patients. Am J Kidney Dis 36:934–944;2000.
Thompson CH, Kemp GJ, Taylor DJ, Ledingham GG, Radda GK, Rajagopalan B. Effect of chronic uremia on skeletal muscle metabolism in man. Nephrol Dial Transplant 8:218–222;1999.
Wallace DC, Zheng X, Lott MT, Shoffner JM, Hodge JA, Kelley FI, Epstein CM, Hopkins LC. Familial mitochondrial encephalomyopathy (MERRF): Genetic, pathophysiological, and biochemical characterization of a mitochondrial DNA disease. Cell 55:601–610;1988.
Wei YH. Oxidative stress and mitochondrial DNA mutations in human aging. Proc Soc Exp Biol Med 217:53–63;1998.
Wei YH, Ding WH, Wei RD. Biochemical effects of PR toxin on rat liver mitochondrial respiration and oxidative phosphorylation. Arch Biochem Biophys 230:400–411;1984.
Wei YH, Kao SH, Lee HC. Simultaneous increase of mitochondrial DNA deletions and lipid peroxidation in human aging. Ann N Y Acad Sci 15;786:24–43;1996.
Wei YH, Lin TN, Hong CY, Chiang BN. Inhibition of the mitochondrial Mg2+-ATPase by propranolol. Biochem Pharmacol 34:911–917;1985.
Witko-Sarsat V, Friedlander M, Capeillere-Blandin C, Nguyen-Khoa T, Nguyen AN, Zingraff J, Jungers P, Descamps-Latscha B. Advanced glycation protein products as a novel marker of oxidative stress in uremia. Kidney Int 49:1304–1313;1996.
Wong SHY, Knight JA, Hopfer SM, Zaharia O, Leach CN, Sunderman FW. Lipoperoxides in plasma as measured by liquid-chromatographic separation of malondialdehyde-thiobarbituric acid adduct. Clin Chem 33:214–220;1987.
Yakes FM, van Houten B. Mitochondrial DNA damage is more extensive and persists longer than nuclear DNA damage in human cells following oxidative stress. Proc Natl Acad Sci USA 94:514–519;1997.
Yu BP, Suescun EA, Yang SY. Effect of age-related lipid peroxidation on membrane fluidity and phospholipase A2 modulation by dietary restriction. Mech Ageing Dev 65:17–33;1992.
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Lim, PS., Ma, YS., Cheng, YM. et al. Mitochondrial DNA mutations and oxidative damage in skeletal muscle of patients with chronic uremia. J Biomed Sci 9, 549–560 (2002). https://doi.org/10.1007/BF02254982
Received:
Accepted:
Issue Date:
DOI: https://doi.org/10.1007/BF02254982