Summary
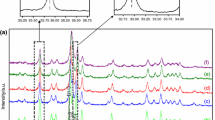
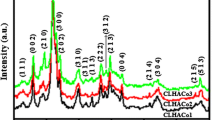
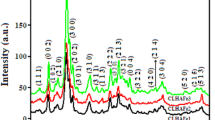
Spectroscopic properties of fluoridated CO3-apatites were studied by means of infrared (IR) absorption analysis. The peak of the IR band caused by CO 2−3 ions at 875 cm−1 shifted to a lower frequency with the degree of fluoridation. This result suggests that there is a significant interaction between F− and CO 2−3 ions in the apatite crystals. The interaction of both these ions is discussed with regard to the solubility behavior of fluoridated CO3-apatites in the acetate buffer solution at pH 4.0 and at 37°C. Solubility of these fluoridated CO3-apatites approached that of fluoridated hydroxyapatites at high fluoride content.
Similar content being viewed by others
References
Corbridge DEC, Lowe EJ (1954) The infra-red spectra of some inorganic phosphorus compounds. Chem Soc 1954:493–502
Emerson WH, Fisher EE (1962) The infra-red absorption spectra of carbonate in calcified tissues. Arch Oral Biol 7:671–683
Berry EE, Baddiel CB (1967) The infra-red spectrum of dicalcium phosphate dihydrate (brushite). Spectrochim Acta 23A:2089–2097
LeGeros RZ, LeGeros JP, Trautz OR, Klein E (1970) Spectral properties of carbonate in carbonate-containing apatites. Dev Appl Sepc 7B:3–12
Klein E, LeGeros JP, Trautz OR, LeGeros RZ (1970) Polarized infrared reflectance of single crystals of apatites. Dev Appl Spec 7B:13–22
Termine JD, Eanes ED (1972) Comparative chemistry of amorphous and apatitic calcium phosphate preparations. Calcif Tissue Res 10:171–197
Bonel G (1972) Contribution a l'étude de la carbonatation des apatites. Ann Chim 7:127–144
Termine JD, Lundy DR (1973) Hydroxide and carbonate in rat bone mineral and its synthetic analogues. Calcif Tissue Res 13:73–82
Termine JD, Lundy DR (1974) Vibrational spectra of some phosphate salts amorphous to x-ray diffraction. Calcif Tissue Res 15:55–70
Amberg CH, Luk HC, Wagstaff KP (1974) The fluoridation of nonstoichiometric calcium hydroxyapatite. An infrared study. Can J Chem 52:4001–4006
Arends J, Davidson CL (1975) HPO 2−4 content in enamel and artificial carious lesions. Calcif Tissue Res 18:65–79
Blumenthal NC, Bett F, Posner AS (1975) Effect of carbonate and biological macromolecules on formation and properties of hydroxyapatite. Calcif Tissue Res 18:81–90
Biltz RM, Pellegrino ED (1977) The nature of bone carbonate. Clin Orthop 129:279–292
LeGeros RZ (1977) Apatites from aqueous and non-aqueous systems: relation to biological apatites. 1st Int. Congr. Phosphorus Compounds, Rabat 1977, pp 347–360
Dowker SEP, Elliot JC (1979) Infrared absorption bands from NCO− and NCN2− in heated carbonate-containing apatites prepared in the presence of NH +4 ions. Calcif Tissue Int 29:177–178
French EL, Welch EA, Simmon ES, LeFevre ML, Hodge HC (1938) Calcium phosphorus and carbon dioxide determination on all the dentin from sound and carious teeth. J Dent Res 17:401–410
Grøn P, Spinelli M, Trautz O, Brudevold F (1963) The effect of carbonate on the solubility of hydroxyapatite. Arch Oral Biol 8:251–263
LeGeros RZ, Trautz OR, LeGeros JP, Klein E (1968) Carbonate substitution in the apatite structure (1). Bull Soc Chim Fr 1968:1712–1718
Okazaki M, Moriwaki Y, Aoba T, Doi Y, Takahashi J (1981) Solubility behavior of CO3 apatites in relation to crystallinity. Caries Res 15:477–483
Brudevold F, McCann HG (1968) Enamel solubility tests and their significance in regard to dental caries. Ann NY Acad Sci 153:20–51
Driessens FCM (1973) Relation between apatite solubility and anti-cariogenic effect of fluoride. Nature 243:420–421
Moreno EC, Kresak M, Zahradnik RT (1974) Fluoridated hydroxyapatite solubility and caries formation. Nature 247:64–65
Moreno EC, Kresak M, Zahradnik RT (1977) Physicochemical aspects of fluoride-apatite systems relevant to the study of dental caries. Caries Res 11 (Suppl. 1):142–171
Verbeeck RMH, Thun HP, Driessens FCM (1980) Solubility behavior of defective fluorapatite and fluorhydroxyapatites. Ber Bunsenges Phys Chem 84:159–163
Okazaki M, Aoba T, Doi Y, Takahashi J, Moriwaki Y (1981) Solubility and crystallinity in relation to fluoride content of fluoridated hydroxyapatites. J Dent Res 60:845–849
Conway EJ (1965) Microdiffusion Analysis and Volumetric Error, 3rd ed. Van Nostrand, New York
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Okazaki, M. F−-CO 2−3 interaction in IR spectra of fluoridated CO3-apatites. Calcif Tissue Int 35, 78–81 (1983). https://doi.org/10.1007/BF02405010
Issue Date:
DOI: https://doi.org/10.1007/BF02405010