Summary
Chronic insulin deficiency, in both man and experimental animal models, has been associated with skeletal alterations, the genesis of which remains unknown. Since cartilage growth and maturation are dependent on the maintenance of adequate glycolytic activity, we evaluated cartilaginous carbohydrate metabolism and epiphyseal growth plate morphology in control, long-term (7 weeks) streptozotocin-induced diabetic and insulintreated diabetic rats. Since parathyroid hormone levels have been shown to be decreased in chronically diabetic rats, we also studied the effect of a low calcium diet (0.1%) on cartilage metabolism and morphology in the insulinopenic state.
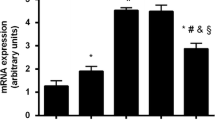
In vitro incubation of epiphyseal cartilage slices in Kreb's Ringer buffer was performed in 5 mM glucose, with either14C-6-glucose as a glycolytic marker or14C-1-glucose as a pentose phosphate pathway marker. While14C-6-glucose uptake was only marginally reduced in diabetic rat cartilage, lactate production was markedly decreased, approximating 42% of control values, and the activity of the pentose phosphate shunt increased (P<0.01). These biochemical alterations were attended by a marked reduction (P<0.005) in the width of epiphyseal growth plates obtained from rats with untreated diabetes.
Both insulin replacement (P<0.001) and dietary calcium restriction (P<0.02) in diabetic animals resulted in a significant increment in the width of epiphyseal growth plates. These morphologic changes were accompanied by a significant (P<0.02) increase in cartilaginous lactate production, in the absence of altered glucose uptake. While insulin treatment corrected glycolysis, it had little effect on the augmented pentose shunt activity, implying stimulation of both these metabolic pathways. Dietary calcium restriction normalized glycolysis and corrected the accelerated activity of the pentose phosphate pathway.
We conclude that chronic insulin deficiency in the growing rat is attended by alterations in cartilaginous carbohydrate metabolism which may relate not only to insulinopenia per se, but also to the relative hypoparathyroidism that characterizes the chronic experimental diabetic state. The accumulated data also suggest that these metabolic derangements may account, at least in part, for the reduced longitudinal bone growth observed in this growing animal model.
Similar content being viewed by others
References
Levin ME, Boisseau VC, Avioli LV (1976) Effects of diabetes mellitus on bone mass in juvenile and adult onset diabetes. N Engl J Med 294:241–245
Santiago JV, McAlister WH, Ratzak SK, Bussman Y, Haymond MW, Shackelford G, Weldon VV (1977) Decreased cortical thickness and osteopenia in children with diabetes mellitus. J Clin Endocrinol Metab 45:845–848
McNair PS, Madsbad S, Christensen MS, Faber OK, Binder C, Transbl I (1979) Bone mineral loss in insulin-treated diabetes mellitus: studies on pathogenesis. Acta Endocrinologica 90:463–472
Heath H, Lambert PW, Service FJ, Arnaud SB (1979) Calcium homeostasis in diabetes mellitus. J Clin Endocrinol Metab 49:462–466
Brown DM, Jowsey J (1977) Osteoporosis in diabetic rats. Diabetes 26:370 (Abstr)
Hough FS, Avioli LV, Bergfeld MA, Fallon MD, Slatopolsky E, Teitelbaum SL (1981) Correction of abnormal bone and mineral metabolism in chronic streptozotocin-induced diabetes mellitus in the rat by insulin therapy. Endocrinology 108:2228–2234
Gutman AB, Yu TFA (1949) A concept of the role of enzymes in endochondral calcification. In: Reifenstein EC, Jr (ed) Metabolic interrelations. Josiah Macy, Jr Foundation, New York, pp 11–26
Picard J, Cartier P (1960) La mineralisation du cartilage ossifiable. X. Glycolyse du cartilage ossifiable de rat jeun normal et de rat rachitique. Bull Soc Chim Biol 42:1117–1123
Borle AB, Nichols N, Nichols G (1960) Metabolic studies of bone in vitro. J Biol Chem 235:1206–1210
Cohn DV, Forscher BK (1962) Aerobic metabolism of glucose by bone. J Biol Chem 237:615–618
Kunin AS, Krane SM (1965) The effect of dietary phosphorus on the intermediary metabolism of epiphyseal cartilage from rachitic rats. Biochim Biophys Acta 107:203–214
Meyer WL, Kunin AS (1969) The inductive effect of rickets on glycolytic enzymes of rat epiphyseal cartilage and its reversal by vitamin D and phosphate. Arch Biochem Biophys 129:438–446
Balogh K, Kunin AS (1968) The effects of vitamin D and dietary phosphorus on oxidative enzymes in the epiphyseal cartilage of rachitic rats. Lab Invest 18:782–788
Kunin AS, Krane SM (1965) Utilization of citrate by epiphyseal cartilage of rachitic and normal rats. Biochim Biophys Acta 111:32–39
Kunin AS, Meyer WL (1969) The effect of cortisone on the intermediary metabolism of epiphyseal cartilage from rats. Arch Biochem Biophys 129:421–430
Meyer WL, Kunin AS (1969) Decreased glycolytic enzyme activity in epiphyseal cartilage of cortisone-treated rats. Arch Biochem Biophys 129:431–437
Balogh K, Kunin AS (1971) The effect of cortisone on the metabolism of epiphyseal cartilage. A histochemical study. Clin Orthop 80:208–215
Russell JE, Avioli LV (1975) Alterations of cartilaginous aerobic glycolysis in the chronic uremic state. Kidney Int 7:333–337
Ganda OM, Rossini AA, Like AA (1976) Studies on streptozotocin diabetes. Diabetes 25:595–603
Keck K (1956) An ultramicro technique for the determination of deoxypentose nucleic acid. Arch Biochem Biophys 63:446–451
Goldberg ND, Passonneau JV, Lowry OH (1966) Effects of changes in brain metabolism on the levels of citric acid cycle intermediates. J Biol Chem 241:3997–4003
Jones R McClung (1950) Handbook of microscopical technique: 3rd ed. Paul B. Hoeber, New York, p 249
merz WA, Schenk RK (1970) Quantitative structural analysis of human cancellous bone. Acta Anat (Basel) 75:54–66
Wood HG, Katz J, Landau BR (1963) Estimation of pathways of carbohydrate metabolism. Biochemistry 338:809–847
Hough S, Slatopolsky E, Avioli LV (1981) Hormonal alterations in experimental diabetes: role of a primary disturbance in calcium homeostasis. Clin Res 29:408A
Bernstein DS, Leboeuf B, Cahill GF (1961) Studies on glucose metabolism in cartilage in cartilage in vitro. Proc Soc Exp Biol Med 107:458–461
Landau BR (1970) Carbohydrate metabolism. In: Ellenberg M and Rifkin H (eds) Diabetes Mellitus: theory and practice. McGraw Hill, New York, pp 2–27
Sochor M, Baquer NZ, McLean P (1979) Glucose overutilization in diabetes: evidence from studies on the changes in hexokinase, the pentose phosphate pathway and glucoronate-xylulose pathway in rat kidney cortex in diabetes. Biochem Biophys Res Comm 86:32–39
Gonzalez AM, Sochor M, Hothersall JS, McLean P (1978) Effect of experimental diabetes on the activity of hexokinase in rat lens: an example of glucose overutilization in diabetes. Biochem Biophys Res Comm 84:858–864
De Nicola AF, Fridman O, Del Castillo EJ, Voglia VG (1977) Abnormal regulation of adrenal function in rats with streptozotocin diabetes. Horm Metab Res 9:469–473
Nichols G, Flanagan B, Woods JF (1965) Parathyroid influences on bone biosynthetic mechanisms. In: Gaillard PJ, Talmage PV, Budy AM (eds) The Parathyroid Glands. University of Chicago Press, Chicago, pp 243–260
Firschein H, Martin G, Mulryan BJ, Strates B, Neuman WF (1958) Concerning the mechanism of action of parathyroid hormone. J Am Chem Soc 80:1619–1623
Borle AB, Nichols N, Nichols G (1960) Metabolic studies of bone in vitro. The metabolic patterns of accretion and resorption. J Biol Chem 235:1211–1214
Martin GR, Mecca CE, Schiffmann E, Goldhaber P (1965) Alterations in bone metabolism induced by parathyroid extract. In: Gaillard PJ, Talmage PV, Budy AM (eds) The parathyroid glands. University of Chicago Press, Chicago, pp 261–272
Cohn DV (1964) Influence of parathyroid extract on the metabolism of organic acids by bone slices. Endocrinology 74:133–137
Gonzalez AM, Sochor M, McLean P (1980) Effect of experimental diabetes on glycolytic intermediates and regulation of phosphofructokinase in rat lens. Biochem Biophys Res Comm 95:1173–1179
Phillips LS, Vassilopoulou-Sellin R (1980) Somatomedins. N Engl J Med 302:438–444
Takano K, Hizuka N, Shizume K, Hasumi Y, Kogawa M, Tsushima T (1980) Effect of insulin and nutrition on serum levels of somatomedin A in the rat. Endocrinology 107:1614–1619
Phillips LS, Orawski AT (1977) Nutrition and somatomedin. III Diabetic control, somatomedin, and growth in rats. Diabetes 26:864–869
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Hough, S., Russell, J.E., Teitelbaum, S.L. et al. Regulation of epiphyseal cartilage metabolism and morphology in the chronic diabetic rat. Calcif Tissue Int 35, 115–121 (1983). https://doi.org/10.1007/BF02405016
Issue Date:
DOI: https://doi.org/10.1007/BF02405016