Summary
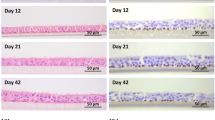
In the present study we describe the establishment of serial cultures of human bronchial epithelial cells derived from biopsies obtained by fiberoptic bronchoscopy. The cell cultures were initiated from small amounts of material (2 mm forceps biopsies) using either explants or epithelial cell suspensions in combination with a feeder-layer technique. The rate of cell proliferation and the number of passages (up to 8 passages) achieved were similar, irrespective of whether the explants or dissociated cells were used. To modulate the extent of differentiation, the bronchial epithelial cells were cultured either under submerged, low calcium (0.06 mM) (proliferating), normal calcium (1.6 mM) (differentiation enhancing) conditions, or at the air-liquid interface. Characterization of the bronchial epithelial cell cultures was assessed on the basis of cell morphology, cytokeratin expression, and ciliary activity. The cells cultured under submerged conditions formed a multilayer consisting of maximally three layers of polygonal-shaped, small cuboidal cells, an appearance resembling the basal cells in vivo. In the air-exposed cultures, the formed multilayer consisted of three to six layers exhibiting squamous metaplasia. The cytokeratin profile in cultured bronchial epithelial cells was similar in submerged and air-exposed cultures and comparable with the profile found in vivo. In addition to cytokeratins, vimentin was co-expressed in a fraction of the subcultured cells. The ciliary activity was observed in primary culture, irrespective of whether the culture had been established from explants or from dissociated cells. This activity was lost upon subculturing and it was not regained by prolongation of the culture period. In contrast to submerged cultures and despite the squamous metaplasia appearance, the cells showed a reappearance of cilia when cultured at the air-liquid interface. Human bronchial epithelial cell cultures can be a representative model for controlling the mechanisms of regulation of bronchial epithelial cell function.
Similar content being viewed by others
References
Barnes, P. J.; Cuss, F. M.; Palmer, J. B. The effect of airway epithelium on smooth muscle contractility in bovine trachea. Br. J. Pharmacol. 86:685–691; 1985.
Broers, J. L. V.; Ramaekers, F. C. S.; Klein Rot, M., et al. Cytokeratins in different types of human lung cancer as monitored by chain-specific monoclonal antibodies. Cancer Res. 48:3221–3229; 1988.
Chopra, D. P.; Sullivan, J.; Wille, J. J., et al. Propagation of differentiating normal human tracheobronchial epithelial cells in serum-free medium. J. Cell. Physiol. 130:173–181; 1987.
Church, M. K.; Lai, C.; Beasley, R., et al. The mediator and cellular basis of the allergic response. Allergy 43 (suppl 8):26–29; 1988.
Crandall, E. D.; Kim, K. J. Protein traffic across lung epithelia. Am. J. Respir. Cell. Mol. Biol. 1:255; 1989.
Dairkee, S. H.; Blayney, C. M.; Asarnow, D. M., et al. Early expression of vimentin in human mammary cultures. In Vitro Cell. Dev. Biol. 21:321–327; 1985.
Flavahan, N. A.; Aarhus, L. L.; Rimele, T. J., et al. Respiratory epithelium inhibits bronchial smooth muscle tone. J. Appl. Physiol. 58:834–838; 1985.
Gray, T. E.; Thomassen, D. G.; Mass, M. J., et al. Quantitation of cell proliferation, colony formation, and carcinogen induced cytotoxicity of rat tracheal epithelial cells grown in culture on 3T3 feeder layers. In Vitro 19:559–570; 1983.
Green, H.; Kehinde, O.; Thomas, J. Growth of cultured human epidermal cells into multiple epithelia suitable for grafting. Proc. Natl. Acad. Sci. USA 76:5665–5668; 1979.
Gruenert, D. C.; Basbaum, C. B.; Widdicombe, J. H. Long-term culture of normal and cystic fibrosis epithelial cells grown under serum-free conditions. In Vitro Cell. Dev. Biol. 26:411–418; 1990.
Hennings, H.; Michael, D.; Cheng, C., et al. Calcium regulation of growth and differentiation of epidermal cells in culture. Cell 19:245–254; 1980.
Hogg, J. C.; Eggleston, P. A. Is asthma an epithelial disease? Am. Rev. Respir. Dis. 129:207–208; 1984.
Holroyde, M. C. The influence of epithelium on the responsiveness of guinea-pig isolated trachea. Br. J. Pharmacol. 87:501–507; 1986.
Ishida, K.; Kelly, L. J.; Thomson, R. J., et al. Repeated antigen challenge induces airway hyperresponsiveness with tissue eosinophilia in guinea pigs. J. Appl. Physiol. 67:1133–1139; 1989.
Jacoby, D. B.; Nadel, J. A. Airway epithelial metabolism and airway smooth muscle hyperresponsiveness. In: Coburn, R. F., ed. Airway smooth muscle in health and disease. New York: Plenum Publishing Corporation; 1989.
Jorissen, M.; Van der Schueren, B.; Van den Berghe, H., et al. Contribution of in vitro culture methods for respiratory epithelial cells to the study of the physiology of the respiratory tract. Eur. Respir. J. 4:210–217; 1991.
Kelsen, S. G.; Mardini, I. A.; Zhou, S., et al. A technique to harvest viable tracheobronchial epithelial cells from living human donors. Am. J. Respir. Cell Mol. Biol. 7:66–72; 1992.
Lane, E. B.; Bártek, J.; Purkis, P. E., et al. Keratin antigens in differentiating skin. Ann. NY Acad. Sci. 455:241–258; 1985.
Lechner, J. F.; Haugen, A.; Autrup, H., et al. Clonal growth of epithelial cells from normal adult human bronchus. Cancer Res. 41:2294–2304; 1981.
Lechner, J. F.; Haugen, A.; McClendon, I. A., et al. Clonal growth of normal adult human bronchial epithelial cells in a serum-free medium. In Vitro 18:633–642; 1982.
Lechner, J. F.; LaVeck, M. A. A serum-free method for culturing normal human bronchial epithelial cells at clonal density. J. Tissue Cult. Methods 9:43–48; 1985.
Liu, S. C.; Karasek, M. Isolation and growth of adult human epidermal keratinocytes in cell culture. J. Invest. Dermatol. 71:157–162; 1978.
Mendelsohn, M. G.; Dilorenzo, T. P.; Abramson, A. L., et al. Retinoic acid regulates, in vitro, the two normal pathways of differentiation of human laryngeal keratinocytes. In Vitro Cell. Dev. Biol. 27A:137–141; 1991.
Moll, R.; Franke, W. W.; Schiller, D. L., et al. The catalog of human cytokeratins: patterns of expression in normal epithelia, tumors and cultured cells. Cell 31:11–24; 1982.
Munakata, M.; Huang, I.; Mitzner, W., et al. Protective role of epithelium in the guinea pig airway. J. Appl. Physiol. 66:1547–1552; 1989.
Nakane, P. K.; Pierce, G. B. Enzyme-labeled antibodies: preparation and application for the localization of antigens. J. Histochem. Cytochem. 14:929–931; 1966.
Oomen, L. C.; Ten Have-Opbroek, A. A.; Hageman, P. C., et al. Fetal mouse alveolar type II cells in culture express several type II cell characteristics found in vivo, together with major histocompatibility antigens. Am. J. Respir. Cell. Mol. Biol. 3:325–339; 1990.
Ponec, M.; Weerheim, A.; Kempenaar, J., et al. Lipid composition of cultured human keratinocytes in relation to their differentiation. J. Lipid Res. 29:949–961; 1988.
Powell, D. W. Barrier function of epithelia. Am. J. Physiol. 241:G275-G288; 1981.
Régnier, M.; Pruniéras, M.; Woodley, D. Growth and differentiation of adult human epidermal cells on dermal substrates. Front. Matrix Biol. 9:4–35; 1981.
Rheinwald, J. G.; Green, H. Serial cultivation of strains of human epidermal keratinocytes. The formation of keratinizing colonies from single cells. Cell 6:331–343; 1975.
Richard, M. H.; Viac, J.; Reano, A., et al. Vimentin expression in normal human keratinocytes grown in serum-free defined MCDB 153 medium. Arch. Dermatol. Res. 282:512–515; 1990.
Salari, H.; Schellenberg, R. R. Stimulation of human airway epithelial cells by platelet activating factor (PAF) and arachidonic acid produces 15-hydroxyeicosatetraenoic acid (15-HETE) capable of contracting bronchial smooth muscle. Pulmonary Pharmacol. 4:1–7; 1991.
Schaafsma, H. E.; Ramaekers, F. C. S.; van Muijen, G. N. P., et al. Distribution of cytokeratin polypeptides in epithelia of the adult human urinary tract. Histochemistry 91:151–159; 1989.
Sigal, E.; Nadel, J. A. The airway epithelium and arachidonic acid 15-lipoxygenase. Am. Rev. Respir. Dis. 143:S71-S74; 1991.
Stuart-Smith, K.; Vanhoutte, P. M. Heterogeneity in the effects of epithelium removal in the canine bronchial tree. J. Appl. Physiol. 63:2510–2515; 1987.
Van Muijen, G. N. P.; Warnaar, S. O.; Ponec, M. Differentiation-related changes of cytokeratin expression in cultured keratinocytes and in fetal, newborn, and adult epidermis. Exp. Cell Res. 171:331–345; 1987.
Van Muijen, G. N. P.; Ruiter, D. J.; Franke, W. W., et al. Cell type heterogeneity of cytokeratin expression in complex epithelia and carcinomas as demonstrated by monoclonal antibodies specific for cytokeratins nos. 4 and 13. Exp. Cell. Res. 162:97–113; 1986.
Watt, F. M. Terminal differentiation of epidermal keratinocytes. Curr. Opinion Cell Biol. 1:1107–1115; 1989.
Wu, R.; Martin, W. R.; Robinson, C. B., et al. Expression of mucin synthesis and secretion in human tracheobronchial epithelial cells grown in culture. Am. J. Respir. Cell Mol. Biol. 3:467–478; 1990.
Wu, R.; Nolan, E.; Turner, C. Expression of tracheal differentiated functions in serum-free, hormone-supplemented medium. J. Cell Physiol. 127:167–181; 1985.
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
de Jong, P.M., van Sterkenburg, M.A.J.A., Kempenaar, J.A. et al. Serial culturing of human bronchial epithelial cells derived from biopsies. In Vitro Cell Dev Biol - Animal 29, 379–387 (1993). https://doi.org/10.1007/BF02633985
Received:
Accepted:
Issue Date:
DOI: https://doi.org/10.1007/BF02633985