Abstract
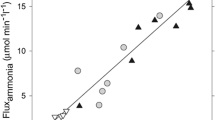
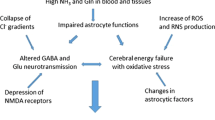
There is substantial clinical and experimental evidence to suggest that ammonia toxicity is a major factor in the pathogenesis of hepatic encephalopathy associated with subacute and chronic liver disease. Ammonia levels in patients with severe liver disease are frequently found to be elevated both in blood and cerebrospinal fluid (csf). Hepatic encephalopathy results in neuropathological damage of a similar nature (Alzheimer type II astrocytosis) to that found in patients with congenital hyperammonemia resulting from inherited defects of urea cycle enzymes. Following portocaval anastomosis in the rat, blood ammonia concentration is increased 2-fold, and brain ammonia is found to be increased 2–3-fold. Administration of ammonia salts or resins to rats with a portocaval anastomosis results in coma and in Alzheimer type II astrocytosis. Since the CNS is devoid of effective urea cycle activity, ammonia removal by brain relies on glutamine formation. Cerebrospinal fluid and brain glutamine are found to be significantly elevated in cirrhotic patients with encephalopathy and in rats following portocaval anastomosis. In both cases, glutamine is found to be elevated in a region-dependent manner. Several mechanisms have been proposed to explain the neurotoxic action of ammonia. Such mechanisms include: (i) Modification of blood-brain barrier transport; (ii) alterations of cerebral energy metabolism; (iii) direct actions on the neuronal membrane; and (iv) decreased synthesis of releasable glutamate, resulting in impaired glutamatergic neurotransmission.
Similar content being viewed by others
References
Adams, R. D. and Foley J. M. (1953) The neurological disorder associated with liver disease, inMetabolic and Toxic Diseases of the Nervous System (Merritt H. H. and Hare C. C., eds), vol. 32, pp. 198–237, Williams and Wilkins, Baltimore, MD.
Butterworth R. F. and Giguère J. F. (1984) Region-selective glutamine changes in the CNS in relation to function in experimental subacute hepatic encephalopathy, inAdvances in Hepatic Encephalopathy and Urea Cycle Diseases (Kleinberger G., Ferenci P., Riederer P., and Thaler H., eds.), Karger, Basel, Switzerland, pp. 394–401.
Butterworth R. F. and Giguère J. F. (1986) Cerebral amino acids in Portal-Systemic Encephalopathy: Lack of evidence for altered γ-aminobutyric acid (GABA) function.Metab. Brain Dis. 1, 221–228.
Butterworth R. F., Giguère J. F., Samii A., and Bergeron M. (1986) Regional glutamate changes in experimental hepatic encephalopathy.Trans. Am. Soc. Neurochem. 17, 242.
Eck N. V. (1877) Ligature of the portal vein.Voen. Med. J. St. Petersburg. (Russ.) 130, 1–2. See translation by C. G. Child,Surgery, Gynecology and Obstetrics, 375–376 (1953).
Gabuzda G. Jr., Phillips G. B., and Davidson C. S. (1952) Reversible toxic manifestations in patients with cirrhosis of the liver given cation-exchange resins.N. Engl. J. Med. 246, 124–130.
Gibson G. E., Zimber A., Krook L., Richardson E. P., Jr., and Visek W. J. (1974) Brain histology and behaviour of mice injected with urease.J. Neuropathol. Exp. Neurol. 33, 201–211.
Giguère J. F. and Butterworth R. F. (1982) Glutamic acid abnormalities in the central nervous system in hepatic encephalopathy.Clin. Biochem. 15, 95–96.
Giguère J. F. and Butterworth R. F. (1984) Amino acid changes in regions of the CNS in relation to function in experimental portal-systemic encephalopathy.Neurochem. Res. 9, 1309–1321.
Guitterez J. A. and Norenberg M. D. (1975) Alzheimer II astrocytosis following methionine sulfoximine.Arch. Neurol. 32, 123–126.
Hahn M., Massen O., Nenki M., and Pavlov J. (1893) Die Ecksche Fistel zwischen der unteren Hohlvene und der Pfortader und ihre Folger für den Organismus.Ach. Exp. Pathol. Pharmakol. 32, 161–270.
Hamberger A., Hedquist B., and Nystrom B. (1979) Ammonium ion inhibition of evoked release of endogenous glutamate from hippocampal slices.J. Neurochem. 33, 1295–1302.
Hindfelt B., Plum F., and Duffy T. E. (1977) Effect of acute ammonia intoxication on cerebral metabolism in rats with portacaval shunts.J. Clin. Invest. 59, 386–396.
Holmin T. and Siesjö B. K. (1974) The effect of porta-caval anastomosis upon the energy state and upon acid-base parameters of the rat brain.J. Neurochem. 22, 403–412.
James J. H., Jeppsson B., Ziparo V., and Fischer J. E. (1979) Hyperammonemia, plasma amino acid imbalance and blood-brain amino acid transport: A unified theory of portal-systemic encephalopathy.Lancet 2, 772–775.
Kvamme E. and Lenda K. (1982) Regulation of glutaminase by exogenous glutamate, ammonia and 2-oxoglutarate in synaptosomal enriched preparation from rat brain.Neurochem. Res. 7, 667–678.
Magnus-Alsleben E. (1920) Über die Bedeutung der Eck'schen Fistel für die normale und pathologische Physiologie der Leber.Ergebn. Physiol. 18, 52–78.
Matthews S. A. (1922) Ammonia, a causative factor in meat poisoning in Eck fistula dogs.Am. J. Physiol. 59, 459–460.
McCandless D. W. (1981) Metabolic turnover in the reticular activating system in ammonia-induced coma.Neurobehav. Toxicol. Teratol. 3, 257–260.
McDermott W. V. and Adams R. D. (1954) Episodic stupor associated with an Eck fistula in the human with particular reference to the metabolism of ammonia.J. Clin. Invest. 33, 1–9.
Meyer H. and Lux H. D. (1974) Action of ammonium on a chloride pump: Removal of hyperpolarizing inhibition in an isolated neuron.Pflug. Arch. Eur. J. Physiol. 350, 185–195.
Norenberg M. D., Lapam L. W., Nichols F. A., and May A. G. (1974) An experimental model for the study of hepatic encephalopathy.Arch. Neurol. 31, 106–109.
Phear E. A., Sherlock S., and Summerskill W. H. J. (1955) Blood ammonium levels in liver disease and hepatic coma.Lancet 7, 836–840.
Plum F. and Hindfelt B. (1976) The neurological complications of liver disease, inHandbook of Clinical Neurology (Vinken P. H. and Bruyn G. W., eds.), vol. 27, pp. 349–377, American Elsevier, New York, NY.
Raabe W. and Gumnit R. J. (1975) Disinhibition in cat motor cortex by ammonia.J. Neurophysiol. 38, 347–355.
Record C. O., Buxton B., Chase R. A., Curzon G., Murray-Lyon I. M., and Williams R. (1976) Plasma and brain amino acids in fulminant hepatic failure and their relationship to hepatic encephalopathy.Eur. J. Clin. Invest.,6, 387–394.
Schenker S. and Hoyumpa A. M. Jr. (1984) Pathophysiology of hepatic encephalopathy.Hosp. Prac. 99–121.
Theoret Y., Davies M. F., Esplin B. and Capek R. (1985) Effects of ammonium chloride on synaptic transmission in the rat hippocampal slice.Neuroscience 14, 798–806.
Traeger H. S., Gabuzda S. J., Ballou A. N., and Davidson C. S. (1954) Blood ammonia concentration in liver disease and liver coma.Metabolism 3, 99–109.
Von Hosselin C. and Alzheimer A. (1912) Ein Beitrag zur klinik und pathologischen anatomie der Westphal-Strumpellschen pseudosklerose.Z. Neurol. Psychiat. 8, 183–209.
Watanabe A., Takei N., Higashi T., Shiota T., Nakatsukasa H., Fujiwara M., Sakata T., and Nagashima H. (1984) Glutamic acid and glutamine levels in serum and cerebrospinal fluid in hepatic encephalopathy.Biochem. Med. 32, 225–231.
Zieve L. (1982) Hepatic Encephalopathy, inDiseases of the Liver, 5th Ed. (Schiff L. and Schiff E. R., eds.), pp. 433–459, Lippincott, Philadelphia, PA.
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Butterworth, R.F., Giguère, JF., Michaud, J. et al. Ammonia: Key factor in the pathogenesis of hepatic encephalopathy. Neurochemical Pathology 6, 1–12 (1987). https://doi.org/10.1007/BF02833598
Received:
Accepted:
Issue Date:
DOI: https://doi.org/10.1007/BF02833598