Abstract
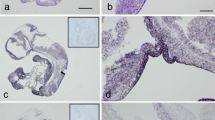
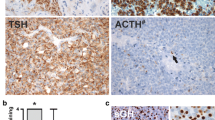
In previous studies, we demonstrated several growth factors, including epidermal growth factor (EGF), transforming growth factorβ (TGFβ), insulin-like growth factor-I (IGF-I, somatomedin C), and TGFe in extracts of the human pituitary. Using immunohistochemistry, reactivity for TGFα, EGF, and TGFβ was localized in sections of 12 of 12 autopsy-derived human pituitary glands. IGF-I reactivity was demonstrated in 5 of 5 pituitary glands. Sections staining for TGFα and IGF-I were double-stained for the full spectrum of anterior pituitary hormones (i.e., GH, PRL, ACTH, LH, FSH, TSH, and a-subunit). Intracellular EGF immunostaining was demonstrated within the posterior pituitary and in some squamous cell nests of the pars tuberalis. Occasional extracellular EGF reactivity was also noted within connective tissue of the anterior pituitary. Polyclonal antibody to TGFβ showed extracellular reactivity within connective tissue surrounding the gland as well as that separating cords of secretory cells. TGFβ staining was also noted in the mediae of small vessels. Monoclonal anti-TGFα antibody labeled scattered secretory cells throughout the anterior pituitary, including ones engaged in follicle formation, and some cells lining Rathke’s cleft remnants. TGFα reactivity in secretory cells was expressed in cells also staining for PRL, α-subunit, LH, FSH, and TSH. No TGFα reactivity was noticed in cells staining for either GH or ACTH. IGF-I reactivity was observed in occasional secretory cells within the anterior pituitary and in neuronal processes of the posterior pituitary. Anterior pituitary cells reactive for hormones were nonreactive for IGF-I. The presence of EGF and TGFβ reactivity within extracellular matrix suggests that it represents a possible storage site of these growth factors within the anterior pituitary. The presence of EGF and IGF-I within the axons and terminations of posterior pituitary implies either local or possibly hypothalamic production of EGF. Finally, our results indicate that TGFα and IGF-I, as well as perhaps EGF, are manufactured by specific pituitary cells and that they may have a role in the endocrine function of the anterior and posterior pituitary, respectively.
Similar content being viewed by others
References
Bach, MA, Bondy CA. Anatomy of the pituitary insulin-like growth factor system. Endocrinology 131:2588–2594, 1992.
Baird A, Mormede P, Ying S-Y, Wehrenberg WB, Ueno N, Ling N, Guillemin R. A nonmitogenic pituitary function of fibroblast growth factor: regulation of thyrotropin and prolactin secretion. Proc Natl Acad Sci USA 82:5545–5449, 1985.
Berg KK, Scheithauer BW, Klee GG, Laws ER Jr, Kovacs K, Horvath E, Preissner BS. Alpha-Subunit in pituitary adenomas: a radioimmunoassay and immunohistochemical study. Adv Biosci 66:397–411, 1988.
Binoux M, Hossenlop P, Lassarre C, Hardouin N. Production of insulin-like growth factors and their carriers by rat pituitary gland and brain explants in culture. FEBS Lett 124:178–184, 1981.
Borgundvaag B, Kudlow JE, Mueller SG, George SR. Dopamine receptor activation inhibits estrogen-stimulated transforming growth factor-a gene expression and growth in anterior pituitary, but not on uterus. Endocrinology 130:3453–3458, 1992.
Brown CA, Halper J. Mitogenic effects of transforming growth factor type e on epithelial and fibroblastic cell lines: comparison with other growth factors. Exp Cell Res 190:233–242, 1990.
Cohen KL, Nissley SP. The serum half-life of somatomedin: evidence for growth hormone dependence. Acta Endocrinol 83:243–258, 1976.
Doi M, Igarashi M, Hasegawa Y, Eto Y, Shibai H, Miura T, Ibuki Y. In vivo action of activin-A on pituitary-gonadal system. Endocrinology 130:139–144, 1991.
Drago J, Murphy M, Carroll SM, Harvey RP, Bartlett PR. Fibroblast growth factormediated proliferation of central nervous system precursors depends on endogenous production of insulin-like growth factor I. Proc Natl Acad Sci USA 88:2199–2203, 1991.
Driman DK, Kobrin MS, Kudlow JE, Asa SL. Transforming growth factor-a in nor- mal and neoplastic human endocrine tissues. Hum Pathol 23:1360–1365, 1992.
Fagin JA, Brown A, Melmed S. Regulation of pituitary insulin-like growth factor-I messenger ribonucleic acid levels in rats harboring somatomammotropic tumors: implications for growth hormone autoregulation. Endocrinology 122:2204–2210, 1988.
Fagin JA, Fernandez-Mejia C, Melmed S. Pituitary insulin-like growth factor-I gene expression: regulation by triiodothyronine and growth hormone. Endocrinology 125: 2385–2391, 1989.
Ferrara N, Schweigerer L, Neufeld G, Mitchell R, Gospodarowicz D. Pituitary follicular cells produce basic fibroblast growth factor. Proc Natl Acad Sci USA 84:5773–5777, 1987.
Franchimont P, Hazee-Hagelstein M-T, Jaspar J-M, Charlet-Renard C, Demoulin A. Inhibin and related peptides: mechanisms of action and regulation of secretion. J Steroid Biochem 32:193–197, 1989.
Gambarini AG, Armelin HA. Purification and partial characterization of an acidic fibroblast growth factor from bovine pituitary. J Biol Chem 257:9692–9697, 1982.
Gospodarowicz D. Purification of a fibroblast growth factor from bovine pituitary. J Biol Chem 250:2515–2520, 1975.
Goustin AS, Leof EB, Shipley GD, Moses HL. Growth factors and cancer. Cancer Res 46:1015–1029, 1986.
Halper J, Pamell PG, Carter BJ, Ren P, Scheithauer BW. Presence of growth factors in human pituitary. Lab Invest 66:639–645, 1992.
Hsu S-M, Raine L. The use of avidinbiotin-peroxidase complex (ABC) in diagnostic and research pathology. In: DeLellis RA ed. Advances in immunohistochemistry. New York: 1984:31-42.
Inoue K, Sakai T, Hattori M. The cell-adhesive effect of basic fibroblast growth factor on pituitary cells in vitro. J Endocrinol 130:381–386, 1991.
Jones TH, Brown BL, Dobson PRM. Paracrine control of anterior pituitary hormone secretion. J Endocrinol 127:5–13, 1990.
Kajikawa K, Yasui W, Sumiyoshi H, Yoshida H, Nakayama H, Ayhan A, Yokozaki H, Ito H, Tahara E. Expression of epidermal growth factor in human tissue. Virchows Arch [A] 418:27–32, 1991.
Kannan CR. LH and FSH: the gonadotropins. In: Kannan CR, ed. Clinical surveys in endocrinology, vol 1. The pituitary gland, vol II. New York: Plenum Medical, 1987:309–327.
Kasselberg AG, Orth DN, Gray ME, Stahlman MT. Immunocytochemical localization of human epidermal growth factor/urogastrone in several human tissues. J Histochem Cytochem 33:315–322, 1985.
Kobrin MS, Asa SL, Samsoondar J, Kudlow JE. α-Transforming growth factor in bovine anterior pituitary gland: secretion by dispersed cells and immunohistochemical localization. Endocrinology 121:1412–1416, 1987.
Mueller SG, Kobrin MS, Paterson AJ, Kudlow JE. Transforming growth factor-α expression in the anterior pituitary gland: regulation by epidermal growth factor and phorbol ester in dispersed cells. Mol Endocrinol 3:976–983, 1989.
Mueller SG, Kudlow JE. Transforming growth factor-β (TGFβ) inhibits TGFα expression in bovine anterior pituitary-derived cells. Mol Endocrinol 5:1439–1446, 1991.
Murata T, Ying S-Y. Transforming growth factor-β and activin inhibit basal secretion of prolactin in a pituitary monolayer culture system. Proc Soc Exp Biol Med 198:599–605, 1991.
Murdoch GH, Potter E, Nicolaisen AK, Evans RM, Rosenfeld MG. Epidermal growth factor rapidly stimulates prolactin gene expression. Nature 300:192–194, 1982.
Newman GR, Jasani B, Williams ED. Multiple storage by cells of the human pituitary. J Histochem Cytochem 37:1183–1192, 1989.
Noguchi T, Sugisaki T, Kanamatsu T, Nishikawa N. Presence of a insulin-like growth factor I (somatomedin C) immunoreactive substance in GH producing cells in the bovine anterior pituitary. Hormone Metabol Res 21:165–167, 1989.
Oberbauer AM, Linkhart TA, Mohan S, Longo LD. Fibroblast growth factor enhances human chorionic gonadotropin synthesis independent of mitogenic stimulation in Jar choriocarcinoma cells. Endocrinology 123:2696–2700, 1988.
Oberbauer AM, Strong DD, Linkhart TA, Mohan S, Longo LD. Fibroblast growth factor enhances the transcription and stability of human chorionic gonadotropin β-subunit messenger ribonucleic acid in Jar choriocarcinoma cells. Endocrinology 132: 757–762, 1993.
Pons S, Torres-Aleman I. Basic fibroblast growth factor modulates insulin-like growth factor-I, its receptor, and its binding proteins in hypothalamic cell cultures. Endocrinology 131:2271–2278, 1992.
Ramsdell JS. Transforming growth factor-α and -β are potent and effective inhibitors of GH4 pituitary tumor cell proliferation. Endocrinology 128:1981–1990, 1991.
Ramsdell JS, Paquette DA, Finley EL. Transforming growth factor-α forms an autocrine growth inhibitory feedback loop in pituitary cells that is under estrogen regulation. J Cell Biochem 16B (Suppl):156, 1992.
Ruoslahti E, Yamaguchi Y. Proteoglycans as modulators of growth factor activities. Cell 64:867–869, 1991.
Salmon WD, Daughaday WH. A hormonally controlled serum factor which stimulates sulfate incorporation by cartilage in vitro. J Lab Clin Med 49:825–836, 1957.
Shome B, Parlow AF. Human follicle stimulating hormone (hFSH): first proposal for the amino acid sequence of the alpha-subunit (hFSH-α) and first demonstration of its identity with the alpha-subunit of human luteinizing hormone (hLH-α). J Clin Endocrinol Metab 39:199–202, 1974.
Sporn MB, Roberts AB. Transforming growth factor-β: multiple actions and potential clinical applications. J Am Med Assoc 262:938–941, 1989.
Tsonis CG, Sharp PJ, McNeilly AS. Inhibin bioactivity and pituitary cell mitogenic activity from cultured chicken ovarian granulosa and thecal/stromal cells. J Endocrinol 116:293–299, 1988.
Yamaguchi Y, Mann DM, Ruoslahti, E. Negative regulation of transforming growth factor-β by the proteoglycan decorin. Nature 346:281–284, 1990.
Ying S-Y, Becker A, Baird A, Ling N, Ueno N, Esch F, Guillemin R. Type beta transforming growth factor (TGF-β) is a potent stimulator of the basal secretion of follicle stimulating hormone (FSH) in a pituitary monolayer system. Biochem Biophys Res Commun 135:950–956, 1986.
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Ren, P., Scheithauer, B.W. & Halper, J. Immunohistological localization of TGFα, EGF, IGF-I, and TGFβ in the normal human pituitary gland. Endocr Pathol 5, 40–48 (1994). https://doi.org/10.1007/BF02921369
Published:
Issue Date:
DOI: https://doi.org/10.1007/BF02921369