Abstract
Objective
Mice and rats are important animal models for mast cell (MC) study. However, rat Mas-related-GPCR-B3 receptor (MRGPRB3) has been less studied than its mouse counterpart. Therefore, we aimed to characterize rat MRGPRB3.
Methods
Mrgprb3 mRNA expression was assessed in peritoneal cells (RPCs) and peritoneal MCs (RPMCs) of wild-type rats, RPCs of MC-deficient rats, and RBL-2H3 cells by reverse-transcriptase polymerase chain reaction (RT-PCR). RPMCs, MRGPRX2-transfected and non-transfected RBL-2H3 cells were activated by 15–30 min incubation with DNP-BSA, substance-P (SP), or compound-48/80. L732138 or CP96344 was used as a tachykinin/neurokinin-1-receptor antagonist. Histamine release from MCs was measured by HPLC fluorometry.
Results
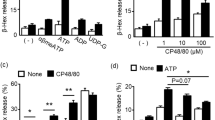
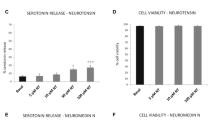
Mrgprb3 mRNA expression was found in all cells, with the highest level in wild-type RPCs. All cells responded to DNP-BSA, but only MRGPRX2-transfected-RBL-2H3 cells and RPMCs responded to all activators. L732138 (0.1–10 μM) and CP96344 (1–100 μM) suppressed SP (10 μM)-induced RPMC activation. L732138 inhibition was dose independent, whereas CP96344 inhibition occurred in a dose-dependent manner. Additionally, only CP96344 suppressed SP (100 μM)- and compound-48/80 (10 μg/mL)-induced RPMC activation.
Conclusions
RPMCs expressing functional MRGPRB3 response upon MRGPRX2 ligands to regulated MC-mediated activities. It`s provide novel insights for future pseudo-allergic studies in rodents.




Similar content being viewed by others
References
Tatemoto K, Nozaki Y, Tsuda R, Konno S, Tomura K, Furuno M, Ogasawara H, Edamura K, Takagi H, Iwamura H, Noguchi M, Naito T. Immunoglobulin E-independent activation of mast cell is mediated by Mrg receptors. Biochem Biophys Res Commun. 2006;349:1322–8.
Mousli M, Bueb JL, Bronner C, Rouot B, Landry Y. G Protein activation: a receptor-independent mode of action for cationic amphiphilic neuropeptides and venom peptides. Trends Pharmacol Sci. 1990;11(9):358–62.
Chahdi A, Fraundorfer PF, Beaven MA. Compound 48/80 activates mast cell phospholipase D via heterotrimeric GTP-binding proteins. J Pharmacol Exp Ther. 2000;292(1):122–30.
Palomaki VA, Laitinen JT. The basic secretagogue compound 48/80 activates G proteins indirectly via stimulation of phospholipase d-lysophosphatidic acid receptor axis and 5-HT1A receptors in rat brain sections. Br J Pharmacol. 2006;147(6):596–606.
Thurmond RL, Gelfand EW, Dunford PJ. The role of histamine H1 and H4 receptors in allergic inflammation: the search for new antihistamines. Nat Rev Drug Disc. 2008;7(1):41–53.
Ohsawa Y, Hirasawa N. The role of histamine H1 and H4 receptors in atopic dermatitis: from basic research to clinical study. Allergol Int. 2014;63:533–42.
Azimi E, Reddy VB, Shade K-TC, Anthony RM, Talbot S, Pereira PJS, Lerner EA. Dual action of neurokinin-1 antagonists on Mas-related GPCRs. JCI Insight. 2016;1(16):e89362.
Dong X, Han S-K, Zylka MJ, Simon MI, Anderson DJ. A diverse family of GPCRs expressed in specific subsets of nociceptive sensory neurons. Cell. 2001;106:619–32.
Liu Q, Tang Z, Surdenikova L, Kim S, Patel KN, Kim A, Ru F, Guan Y, Weng H-J, Geng Y, Undem BJ, Kollarik M, Chen Z-F, Anderson DJ, Dong X. Sensory neuron-specific GPCR Mrgprs are itch receptors mediating chloroquine-induced pruritus. Cell. 2009;139:1353–65.
Robas N, Mead E, Fidock M. MrgX2 is a high potency cortistatin receptor expressed in dorsal root ganglion. J Biol Chem. 2003;278(45):44400–4.
Kamohara M, Matsuo A, Takasaki J, Kohda M, Matsumoto M, Matsumoto S, Soga T, Hiyama H, Kobori M, Katou M. Identification of MrgX2 as a human G-protein-coupled receptor for proadrenomedullin N-terminal peptides. Biochem Biophys Res Commun. 2005;330:1146–52.
Nothacker H-P, Wang Z, Zeng H, Mahata SK, O’Connor DT, Civelli O. Proadrenomedullin N-terminal peptide and cortistatin activation of MrgX2 receptor is based on a common structural motif. Eur J Pharmacol. 2005;519:191–3.
McNeil BD, Pundir P, Meeker S, Han L, Undem BJ, Kulka M, Dong X. Identification of a mast-cell-specific receptor crucial for pseudo-allergic drug reactions. Nature. 2015;519(7542):237–41.
Zhang T, Che D, Liu R, Han S, Wang N, Zhang Y, Pundir P, Cao J, Lv Y, Yang L, Wang J, Ding M, Dong X, He L. Typical antimicrobials induced mast cell degranulation and anaphylactoid reactions via MrgprX2 and its murine homologue Mrgprb2. Eur J Immunol. 2017;47(11):1949–58.
Pennefather JN, Lecci A, Candenas ML, Patak E, Pinto FM, Maggi CA. Tachykinins and tachykinin receptors: a growing family. Life Sci. 2004;74:1445–633.
Fridmanis D, Roga A, Klovins J. ACTH receptor (MC2R) specificity: what do we know about underlying molecular mechanisms? Front Endocrinol. 2017;8:13.
Tusnady GE, Simon I. Principles governing amino acid composition of integral membrane proteins: application to topology prediction. J Mol Biol. 1998;283:489–506.
Tusnady GE, Simon I. The HMMTOP transmembrane topology prediction server. Bioinformatics. 2001;17:849–50.
Omasits U, Ahrens CH, Muller S, Wollscheid B. Protter: interactive protein feature visualization and integration with experimental proteomic data. Bioinformatics. 2014;30(6):884–6.
Skrabanek L, Campagne F, Weinstein H. Building protein diagrams on the web with the residue-based diagram editor RbDe. Nucleic Acids Res. 2003;31:3856–8.
Kiyoi T, Liu S, Sahid MNA, Shudou M, Ogasawara M, Mogi M, Maeyama K. Morphological and functional analysis of beige (Chediak–Higashi syndrome) mouse mat cells with giant granules. Int Immunopharmacol. 2019;69:202–12.
Yamatodani A, Fukuda H, Iwaeda T, Watanabe T, Wada H. HPLC determination of plasma and brain histamine without previous purification of biological samples: cation exchange chromatography coupled with post-column derivatization fluorometry. J Chromatogr. 1985;344:115–23.
Aun MV, Bonamichi-Santos R, Arantes-Costa FM, Kalil J, Giavina-Bianchi P. Animal models of asthma: utility and limitations. J Asthma Allergy. 2017;10:293–301.
Reber LL, Marichal T, Galli SJ. New models for analyzing mast cell function in vivo. Trends Immunol. 2012;33(12):613–25.
Lei Y, Gregory JA, Nilsson GP, Adner M. Insights into mast cell functions in asthma using mouse models. Pulm Pharmacol Ther. 2013;26(5):532–9.
Kucharewicz I, Bodzenta-Lukaszyk A, Buczko W. Experimental asthma in rats. Pharmacol Rep. 2008;60(6):783–8.
Oka T, Kalesnikoff J, Starkl P, Tsai M, Galli SJ. Evidence questioning cromolyn’s effectiveness and selectivity as a “mast cell stabilizer” in mice. Lab Invest. 2012;92(10):1472–82.
Arizono N, Kasugai T, Yamada M, Okada M, Morimoto M, Tei H, Newlands GF, Miller HR, Kitamura Y. Infection of Nippostrongylus brasiliensis induces development of mucosal-type but not connective tissue-type mast cells in genetically mast cell-deficient Ws/Ws rats. Blood. 1993;81(10):2572–8.
Barsumian EL, Isersky C, Petrino MG, Siraganian RP. IgE-induced histamine release from rat basophilic leukemia cell lines: isolation of releasing and non-releasing clones. Eur J Immunol. 1981;11:317–23.
Passante E, Frankish N. The RBL-2H3 cell line: its provenance and suitability as a model for the mast cell. Inflamm Res. 2009;58:737–45.
Lai J-P, Lai S, Tuluc F, Tansky MF, Kilpatrick LE, Leeman SE, Douglas SD. Differences in the length of the carboxyl terminus mediate functional properties of neurokinin-1 receptor. Proc Natl Acad Sci USA. 2008;105(34):12605–10.
Snider RM, Longo KP, Drozda SE, Lowe JA III, Leeman SE. Effect of CP-96345, a nonpeptide substance P receptor antagonist, on salivation in rats. Proc Natl Acad Sci USA. 1991;88:10042–4.
Griesbacher T, Donnerer J, Legat FJ, Lembeck F. CP-96345, a non-peptide antagonist of substance P: II. Actions on substance P-induced hypotension and bronchoconstriction, and on depressor reflexes in mammals. Naunyn-Schmiedeberg’s Arch Pharmacol. 1992;346:323–7.
Yashpal K, Radhakrishnan V, Coderres TJ, Henry JL. CP-96345, but not its stereoisomer, CP-96344, blocks the nociceptive responses to intrathecally administered substance P and to noxious thermal and chemical stimuli in the rat. Neuroscience. 1993;52(4):1039–47.
Jacob S, Deyo DJ, Cox RA, Jacob RK, Herndon DN, Traber DL, Hawkins HK. Substance P antagonist CP-96345 blocks lung vascular leakage and inflammation more effectively than its stereoisomer CP-96344 in a mouse model of smoke inhalation and burn injury. Toxical Mech Meth. 2010;20(4):197–203.
Leffler A, Ahlstedt I, Engberg S, Svensson A, Billger M, Oberg L, Bjursell MK, Lindstrom E, von Mentzer B. Characterization of species-related differences in the pharmacology of tachykinin NK receptors 1, 2 and 3. Biochem Pharmacol. 2009;77(9):1522–30.
Calcieri MA, Macleod AM, Underwood D, Shiao LL, Ber E, Sadowski S, Yu H, Merchant KJ, Swain CJ, Strader CD, Fong TM. Characterization of the interaction of N-acyl-l-tryptophan benzyl ester neurokinin antagonists with the human neurokinin-1 receptor. J Biol Chem. 1994;269(9):6587–91.
Acknowledgements
Thanks to Ms. Takemasa Erika and Dr. Takeshi Kiyoi for their technical support.
Author information
Authors and Affiliations
Contributions
Conceptualization: MNAS, KM; methodology: MNAS, KM; investigation: MNAS, SL; writing—original draft: MNAS; writing—review and editing: MNAS, KM; supervision: KM, MM.
Corresponding author
Ethics declarations
Conflict of interest
We do not have conflict of interest to disclose.
Additional information
Responsible Editor: John Di Battista.
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
Below is the link to the electronic supplementary material.
11_2020_1319_MOESM3_ESM.pdf
Supplementary Fig. 1 Prediction of transmembrane region of MRGPR family analyzed by HMMTP version 2.0 (http://www.enzim.hu/hmmtop/). (A) Mouse MRGPRB2 protein (NCBI Accession: EDL22965), (B) human MRGPRX2 protein (NCBI Accession: ACG60653), (C) rat MRGPRB3 protein (NCBI Accession: XP_001077174.2). Upper case (O): indicating outer tail region; lower case (o): outer loop or outer tail that close to the cell membrane; upper case (H): transmembrane region; upper case (I): intracellular tail region; and lower case (i): intracellular loop or intracellular tail that close to the cell membrane (PDF 1958 kb)
11_2020_1319_MOESM4_ESM.pdf
Supplementary Fig. 2 Prediction of transmembrane region of MRGPR family analyzed by Protter version 1.0 (http://wlab.ethz.ch/protter/). (A) Mouse MRGPRB2 protein (Uniprot ID: Q3KNA1), (B) human MRGPRX2 protein (Uniprot ID: Q96LB1), and (C) rat MRGPRB3 protein (Uniprot ID: F1LLV4) (PDF 126 kb)
11_2020_1319_MOESM5_ESM.pdf
Supplementary Fig. 3 Prediction of transmembrane region of MRGPR family analyzed by RbDe (http://icb.med.cornell.edu/services/rbde/diagrams). (A) Mouse MRGPRB2 protein (Uniprot ID: Q3KNA1), (B) human MRGPRX2 protein (Uniprot ID: Q96LB1), and (C) rat MRGPRB3 protein (Uniprot ID: F1LLV4) (PDF 2399 kb)
11_2020_1319_MOESM6_ESM.pdf
Supplementary Fig. 4 Histamine release induced by compound-48/80 and SP. Substance-P dose-dependently (1-1000 µM) increased histamine release in RPMCs (a). High concentration compound-48/80 (100 µg/mL) induced histamine release in RBL-2H3 cells (b). Data are expressed as mean + S.E.M. from triplicate experiments. (#) Indicates significant difference vs lower dose group, p < 0.0001 (PDF 38 kb)
11_2020_1319_MOESM7_ESM.pdf
Supplementary Fig. 5 High concentration of L732138 and CP96344 increases histamine release from RPMCs. Low concentration of TACR1 antagonist L732138 (up to 10 µM) did not induce histamine release from RPMCs (a). Low concentration of TACR1 antagonist CP96344 (up to 100 µM) did not induce histamine release from RPMCs (b). Data are expressed as mean + S.E.M. from triplicate experiments. (#) Indicates significant difference vs lower dose group with p < 0.0001, (##) p < 0.001 (PDF 34 kb)
11_2020_1319_MOESM8_ESM.pdf
Supplementary Fig. 6 Ciprofloxacin induces histamine release from RPMCs. Ciprofloxacin-induced histamine release followed the method for MC activation by compound 48/80, as described in the Methods section. Data are expressed as mean + S.E.M. from triplicate experiments. (#) Indicates significant difference vs dose group, p < 0.0001 (PDF 25 kb)
11_2020_1319_MOESM9_ESM.pdf
Supplementary Fig. 7 Structure of tachykinin receptor 1 (TACR1) antagonists L732138 (a) and CP96344 (b). Box with dashed line highlights the structure mimicking tetrahydroisoquinoline (THIQ) (PDF 165 kb)
Rights and permissions
About this article
Cite this article
Sahid, M.N.A., Liu, S., Mogi, M. et al. Tachykinin-1 receptor antagonism suppresses substance-P- and compound 48/80-induced mast cell activation from rat mast cells expressing functional mas-related GPCR B3. Inflamm. Res. 69, 289–298 (2020). https://doi.org/10.1007/s00011-020-01319-z
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00011-020-01319-z