Abstract
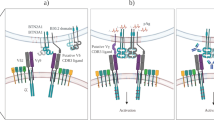
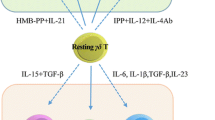
The series of seminal articles in this book clearly illustrate the multi-functional nature of γδ T cells. Some of the functions correlate with the tissue tropism of distinct γδ T cell subsets whereas others appear to result from oligoclonal selection. Here, we discuss the antigen-presenting cell (APC) function of the major subset of circulating γδ T cells, Vγ9/Vδ2 T cells, present in human blood. During tissue culture, Vγ9/Vδ2 T cells uniformly respond to a class of non-peptide antigens, so-called prenyl pyrophosphates, derived from stressed host cells or from microbes. It is this feature that distinguishes human (and primate) Vγ9/Vδ2 T cells from αβ and γδ T cells of all other species and that forms the basis for detailed studies of human Vγ9/Vδ2 T cells. One of the consequences of Vγ9/Vδ2 T cell activation is the rapid acquisition of APC characteristics (γδ T-APCs) reminiscent of mature dendritic cells (DCs). In the following discussion, we will discriminate between the potential use of γδ T-APCs as a cellular vaccine in immunotherapy and their role in anti-microbial immunity. Exploiting the APC function in γδ T-APCs represents a true novelty in current immunotherapy research and may lead to effective, anti-tumor immunity in cancer patients.




Similar content being viewed by others
Abbreviations
- APC:
-
Antigen-presenting cells
- γδ T-APC:
-
Antigen-presenting γδ T cells
- DC:
-
Dendritic cells
- MHC:
-
Major histocompatibility complex antigen
- TCR:
-
T cell antigen receptor
- HMB-PP:
-
(E)-4-hydroxy-3-methyl-but-2-enyl pyrophosphate
- IPP:
-
Isopentenyl pyrophosphate
References
Caccamo N, Dieli F, Wesch D, Jomaa H, Eberl M (2006) Sex-specific phenotypical and functional differences in peripheral human Vgamma9/Vdelta2 T cells. J Leukoc Biol 79:663–666
Morita CT, Jin C, Sarikonda G, Wang H (2007) Nonpeptide antigens, presentation mechanisms, and immunological memory of human Vgamma2Vdelta2 T cells: discriminating friend from foe through the recognition of prenyl pyrophosphate antigens. Immunol Rev 215:59–76
Sicard H, Fournie JJ (2000) Metabolic routes as targets for immunological discrimination of host and parasite. Infect Immun 68:4375–4377
Hintz M, Reichenberg A, Altincicek B, Bahr U, Gschwind RM, Kollas AK, Beck E, Wiesner J, Eberl M, Jomaa H (2001) Identification of (E)-4-hydroxy-3-methyl-but-2-enyl pyrophosphate as a major activator for human gammadelta T cells in Escherichia coli. FEBS Lett 509:317–322
Eberl M, Hintz M, Reichenberg A, Kollas AK, Wiesner J, Jomaa H (2003) Microbial isoprenoid biosynthesis and human gammadelta T cell activation. FEBS Lett 544:4–10
Brandes M, Willimann K, Lang AB, Nam KH, Jin C, Brenner MB, Morita CT, Moser B (2003) Flexible migration program regulates gamma delta T-cell involvement in humoral immunity. Blood 102:3693–3701
Glatzel A, Wesch D, Schiemann F, Brandt E, Janssen O, Kabelitz D (2002) Patterns of chemokine receptor expression on peripheral blood gamma delta T lymphocytes: strong expression of CCR5 is a selective feature of V delta 2/V gamma 9 gamma delta T cells. J Immunol 168:4920–4929
Cipriani B, Borsellino G, Poccia F, Placido R, Tramonti D, Bach S, Battistini L, Brosnan CF (2000) Activation of C-C β-chemokines in human peripheral blood gammaδ T cells by isopentenyl pyrophosphate and regulation by cytokines. Blood 95:39–47
Poggi A, Carosio R, Fenoglio D, Brenci S, Murdaca G, Setti M, Indiveri F, Scabini S, Ferrero E, Zocchi MR (2004) Migration of V delta 1 and V delta 2 T cells in response to CXCR3 and CXCR4 ligands in healthy donors and HIV-1-infected patients: competition by HIV-1 Tat. Blood 103:2205–2213
Moser B, Loetscher P (2001) Lymphocyte traffic control by chemokines. Nat Immunol 2:123–128
Moser B, Wolf M, Walz A, Loetscher P (2004) Chemokines: multiple levels of leukocyte migration control. Trends Immunol 25:75–84
Eberl M, Moser B (2009) Monocytes and gammadelta T cells: close encounters in microbial infection. Trends Immunol 30:562–568
Sallusto F, Mackay CR, Lanzavecchia A (2000) The role of chemokine receptors in primary, effector, and memory immune responses. Annu Rev Immunol 18:593–620
Groh V, Porcelli S, Fabbi M, Lanier LL, Picker LJ, Anderson T, Warnke RA, Bhan AK, Strominger JL, Brenner MB (1989) Human lymphocytes bearing T cell receptor gamma/delta are phenotypically diverse and evenly distributed throughout the lymphoid system. J Exp Med 169:1277–1294
Dieli F, Poccia F, Lipp M, Sireci G, Caccamo N, Di Sano C, Salerno A (2003) Differentiation of effector/memory Vdelta2 T cells and migratory routes in lymph nodes or inflammatory sites. J Exp Med 198:391–397
Vermijlen D, Ellis P, Langford C, Klein A, Engel R, Willimann K, Jomaa H, Hayday AC, Eberl M (2007) Distinct cytokine-driven responses of activated blood gammadelta T cells: insights into unconventional T cell pleiotropy. J Immunol 178:4304–4314
Caccamo N, Battistini L, Bonneville M, Poccia F, Fournie JJ, Meraviglia S, Borsellino G, Kroczek RA, La Mendola C, Scotet E, Dieli F, Salerno A (2006) CXCR5 identifies a subset of Vgamma9 Vdelta2 T cells which secrete IL-4 and IL-10 and help B cells for antibody production. J Immunol 177:5290–5295
Schaerli P, Willimann K, Lang AB, Lipp M, Loetscher P, Moser B (2000) CXC chemokine receptor 5 expression defines follicular homing T cells with B cell helper function. J Exp Med 192:1553–1562
Vinuesa CG, Tangye SG, Moser B, Mackay CR (2005) Follicular B helper T cells in antibody responses and autoimmunity. Nat Rev Immunol 5:853–865
Brandes M, Willimann K, Moser B (2005) Professional antigen-presentation function by human gammadelta T cells. Science 309:264–268
Meuter S, Eberl M, Moser B (2010) Prolonged antigen survival and cytosolic export in cross-presenting human gammadelta T cells. Proc Natl Acad Sci USA 107:8730–8735
Brandes M, Willimann K, Bioley G, Levy N, Eberl M, Luo M, Tampe R, Levy F, Romero P, Moser B (2009) Cross-presenting human gammadelta T cells induce robust CD8+ alphabeta T cell responses. Proc Natl Acad Sci USA 106:2307–2312
Wu Y, Wu W, Wong WM, Ward E, Thrasher AJ, Goldblatt D, Osman M, Digard P, Canaday DH, Gustafsson K (2009) Human gamma delta T cells: a lymphoid lineage cell capable of professional phagocytosis. J Immunol 183:5622–5629
Landmeier S, Altvater B, Pscherer S, Juergens H, Varnholt L, Hansmeier A, Bollard CM, Moosmann A, Bisping G, Rossig C (2009) Activated human gammadelta T cells as stimulators of specific CD8+ T-cell responses to subdominant Epstein Barr virus epitopes: potential for immunotherapy of cancer. J Immunother 32:310–321
Langenkamp A, Messi M, Lanzavecchia A, Sallusto F (2000) Kinetics of dendritic cell activation: impact on priming of TH1, TH2 and nonpolarized T cells. Nat Immunol 1:311–316
Cresswell P, Ackerman AL, Giodini A, Peaper DR, Wearsch PA (2005) Mechanisms of MHC class I-restricted antigen processing and cross-presentation. Immunol Rev 207:145–157
Villadangos JA, Heath WR, Carbone FR (2007) Outside looking in: the inner workings of the cross-presentation pathway within dendritic cells. Trends Immunol 28:45–47
Villadangos JA, Shortman K (2010) Found in translation: the human equivalent of mouse CD8+ dendritic cells. J Exp Med 207:1131–1134
Delamarre L, Pack M, Chang H, Mellman I, Trombetta ES (2005) Differential lysosomal proteolysis in antigen-presenting cells determines antigen fate. Science 307:1630–1634
Davey MS, Lin CY, Roberts GW, Heuston S, Brown AC, Chess JA, Toleman MA, Gahan CG, Hill C, Parish T, Williams JD, Davies SJ, Johnson DW, Topley N, Moser B, Eberl M (2011) Human neutrophil clearance of bacterial pathogens triggers anti-microbial gammadelta T cell responses in early infection. PLoS Pathog 7:e1002040
Eberl M, Roberts GW, Meuter S, Williams JD, Topley N, Moser B (2009) A Rapid crosstalk of human gammadelta T Cells and monocytes drives the acute inflammation in bacterial infections. PLoS Pathog 5:e1000308
Dieli F, Sireci G, Di Sano C, Champagne E, Fournie JJ, Salerno JI (1999) Predominance of Vgamma9/Vdelta2 T lymphocytes in the cerebrospinal fluid of children with tuberculous meningitis: reversal after chemotherapy. Mol Med 5:301–312
Parker CM, Groh V, Band H, Porcelli SA, Morita C, Fabbi M, Glass D, Strominger JL, Brenner MB (1990) Evidence for extrathymic changes in the T cell receptor gamma/delta repertoire. J Exp Med 171:1597–1612
Green AE, Lissina A, Hutchinson SL, Hewitt RE, Temple B, James D, Boulter JM, Price DA, Sewell AK (2004) Recognition of nonpeptide antigens by human V gamma 9 V delta 2 T cells requires contact with cells of human origin. Clin Exp Immunol 136:472–482
Sarikonda G, Wang H, Puan KJ, Liu XH, Lee HK, Song Y, Distefano MD, Oldfield E, Prestwich GD, Morita CT (2008) Photoaffinity antigens for human gammadelta T cells. J Immunol 181:7738–7750
Van Laethem F, Sarafova SD, Park JH, Tai X, Pobezinsky L, Guinter TI, Adoro S, Adams A, Sharrow SO, Feigenbaum L, Singer A (2007) Deletion of CD4 and CD8 coreceptors permits generation of alphabetaT cells that recognize antigens independently of the MHC. Immunity 27:735–750
Pechhold K, Wesch D, Schondelmaier S, Kabelitz D (1994) Primary activation of V gamma 9-expressing gamma delta T cells by Mycobacterium tuberculosis. Requirement for Th1-type CD4 T cell help and inhibition by IL-10. J Immunol 152:4984–4992
Boullier S, Poquet Y, Debord T, Fournie JJ, Gougeon ML (1999) Regulation by cytokines (IL-12, IL-15, IL-4 and IL-10) of the Vgamma9 Vdelta2 T cell response to mycobacterial phosphoantigens in responder and anergic HIV-infected persons. Eur J Immunol 29:90–99
Eberl M, Altincicek B, Kollas AK, Sanderbrand S, Bahr U, Reichenberg A, Beck E, Foster D, Wiesner J, Hintz M, Jomaa H (2002) Accumulation of a potent gammadelta T-cell stimulator after deletion of the lytB gene in Escherichia coli. Immunology 106:200–211
Rojas RE, Torres M, Fournie JJ, Harding CV, Boom WH (2002) Phosphoantigen presentation by macrophages to Mycobacterium tuberculosis–reactive Vgamma9 Vdelta2 + T cells: modulation by chloroquine. Infect Immun 70:4019–4027
Wei H, Huang D, Lai X, Chen M, Zhong W, Wang R, Chen ZW (2008) Definition of APC presentation of phosphoantigen (E)-4-hydroxy-3-methyl-but-2-enyl pyrophosphate to Vgamma2 Vdelta 2 TCR. J Immunol 181:4798–4806
Huang D, Shen Y, Qiu L, Chen CY, Shen L, Estep J, Hunt R, Vasconcelos D, Du G, Aye P, Lackner AA, Larson M, Jacobs WR Jr, Haynes BF, Letvin NL, Chen ZW (2008) Immune distribution and localization of phosphoantigen-specific V{gamma}2 V{delta}2 T cells in lymphoid and non-lymphoid tissues in M tuberculosis infection. Infect Immun 76:426–436
Shen Y, Zhou D, Qiu L, Lai X, Simon M, Shen L, Kou Z, Wang Q, Jiang L, Estep J, Hunt R, Clagett M, Sehgal PK, Li Y, Zeng X, Morita CT, Brenner MB, Letvin NL, Chen ZW (2002) Adaptive immune response of Vgamma2 Vdelta2 + T cells during mycobacterial infections. Science 295:2255–2258
Cairo C, Hebbeler AM, Propp N, Bryant JL, Colizzi V, Pauza CD (2007) Innate-like gammadelta T cell responses to mycobacterium Bacille Calmette-Guerin using the public V gamma 2 repertoire in Macaca fascicularis. Tuberculosis (Edinb) 87:373–383
Cendron D, Ingoure S, Martino A, Casetti R, Horand F, Romagne F, Sicard H, Fournie JJ, Poccia F (2007) A tuberculosis vaccine based on phosphoantigens and fusion proteins induces distinct gammadelta and alphabeta T cell responses in primates. Eur J Immunol 37:549–565
Cheng L, Cui Y, Shao H, Han G, Zhu L, Huang Y, O’Brien RL, Born WK, Kaplan HJ, Sun D (2008) Mouse gammadelta T cells are capable of expressing MHC class II molecules, and of functioning as antigen-presenting cells. J Neuroimmunol 203:2–11
Collins RA, Werling D, Duggan SE, Bland AP, Parsons KR, Howard CJ (1998) Gammadelta T cells present antigen to CD4+ alphabeta T cells. J Leukoc Biol 63:707–714
Takamatsu HH, Denyer MS, Wileman TE (2002) A sub-population of circulating porcine gammadelta T cells can act as professional antigen presenting cells Vet. Immunol Immunopathol 87:223–224
Takamatsu HH, Denyer MS, Stirling C, Cox S, Aggarwal N, Dash P, Wileman TE, Barnett PV (2006) Porcine gammadelta T cells: possible roles on the innate and adaptive immune responses following virus infection. Vet Immunol Immunopathol 112:49–61
Lanzavecchia A, Roosnek E, Gregory T, Berman P, Abrignani S (1988) T cells can present antigens such as HIV gp120 targeted to their own surface molecules. Nature 334:530–532
Price SJ, Hope JC (2009) Enhanced secretion of interferon-gamma by bovine gammadelta T cells induced by coculture with Mycobacterium bovis-infected dendritic cells: evidence for reciprocal activating signals. Immunology 126:201–208
Steinman RM, Banchereau J (2007) Taking dendritic cells into medicine. Nature 449:419–426
Sallusto F, Lanzavecchia A (1994) Efficient presentation of soluble antigen by cultured human dendritic cells is maintained by granulocyte/macrophage colony-stimulating factor plus interleukin 4 and downregulated by tumor necrosis factor alpha. J Exp Med 179:1109–1118
Kondo M, Sakuta K, Noguchi A, Ariyoshi N, Sato K, Sato S, Sato K, Hosoi A, Nakajima J, Yoshida Y, Shiraishi K, Nakagawa K, Kakimi K (2008) Zoledronate facilitates large-scale ex vivo expansion of functional gammadelta T cells from cancer patients for use in adoptive immunotherapy. Cytotherapy 10:842–856
Reid IR, Gamble GD, Mesenbrink P, Lakatos P, Black M (2010) Characterization of and risk factors for the acute-phase response after zoledronic acid. J Clin Endocrinol Metab (in press)
Coleman R (2010) The use of bisphosphonates in cancer treatment. Ann N Y Acad Sci
Kunzmann V, Bauer E, Wilhelm M (1999) Gamma/delta T-cell stimulation by pamidronate. N Engl J Med 340:737–738
Dieli F, Gebbia N, Poccia F, Caccamo N, Montesano C, Fulfaro F, Arcara C, Valerio MR, Meraviglia S, Di Sano C, Sireci G, Salerno A (2003) Induction of gammadelta T-lymphocyte effector functions by bisphosphonate zoledronic acid in cancer patients in vivo. Blood 102:2310–2311
Roelofs AJ, Jauhiainen M, Monkkonen H, Rogers MJ, Monkkonen J, Thompson K (2009) Peripheral blood monocytes are responsible for gammadelta T cell activation induced by zoledronic acid through accumulation of IPP/DMAPP. Br J Haematol 144:245–250
Hu X, Ivashkiv LB (2009) Cross-regulation of signaling pathways by interferon-gamma: implications for immune responses and autoimmune diseases. Immunity 31:539–550
Wesch D, Glatzel A, Kabelitz D (2001) Differentiation of resting human peripheral blood gamma delta T cells toward Th1- or Th2-phenotype. Cell Immunol 212:110–117
Ness-Schwickerath KJ, Jin C, Morita CT (2010) Cytokine requirements for the differentiation and expansion of IL-17A- and IL-22-producing human Vgamma2Vdelta2 T cells. J Immunol 184:7268–7280
Pietschmann K, Beetz S, Welte S, Martens I, Gruen J, Oberg HH, Wesch D, Kabelitz D (2009) Toll-like receptor expression and function in subsets of human gammadelta T lymphocytes. Scand J Immunol 70:245–255
Kobayashi H, Tanaka Y, Yagi J, Osaka Y, Nakazawa H, Uchiyama T, Minato N, Toma H (2007) Safety profile and anti-tumor effects of adoptive immunotherapy using gamma-delta T cells against advanced renal cell carcinoma: a pilot study. Cancer Immunol Immunother 56:469–476
Kobayashi H, Tanaka Y, Shimmura H, Minato N, Tanabe K (2010) Complete remission of lung metastasis following adoptive immunotherapy using activated autologous gammadelta T-cells in a patient with renal cell carcinoma. Anticancer Res 30:575–579
Bennouna J, Bompas E, Neidhardt EM, Rolland F, Philip I, Galea C, Salot S, Saiagh S, Audrain M, Rimbert M, Lafaye-de Micheaux S, Tiollier J, Negrier S (2008) Phase-I study of Innacell gammadelta, an autologous cell-therapy product highly enriched in gamma9delta2 T lymphocytes, in combination with IL-2, in patients with metastatic renal cell carcinoma. Cancer Immunol Immunother 57:1599–1609
Nakajima J, Murakawa T, Fukami T, Goto S, Kaneko T, Yoshida Y, Takamoto S, Kakimi K (2010) A phase I study of adoptive immunotherapy for recurrent non-small-cell lung cancer patients with autologous gammadelta T cells. Eur J Cardiothorac Surg 37:1191–1197
Kunzmann V, Bauer E, Feurle J, Weissinger F, Tony HP, Wilhelm M (2000) Stimulation of gammadelta T cells by aminobisphosphonates and induction of antiplasma cell activity in multiple myeloma. Blood 96:384–392
Lamb LS Jr, Lopez RD (2005) Gammadelta T cells: a new frontier for immunotherapy? Biol Blood Marrow Transplant 11:161–168
June CH, Blazar BR, Riley JL (2009) Engineering lymphocyte subsets: tools, trials and tribulations. Nat Rev Immunol 9:704–716
Acknowledgments
Research has been supported by Grants from the Swiss National Science Foundation, European FP6 (MAIN-NoE, INNOCHEM), Welsh Office for Research and Development, Wellcome Trust, Cancer Research UK, Breast Cancer Campaign, Baxter Healthcare, and Cardiff University i3-IRG. M.E. is a RCUK Fellow in Translational Research in Experimental Medicine; B.M. is the recipient of a Royal Society Wolfson Research Merit Award.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Moser, B., Eberl, M. γδ T-APCs: a novel tool for immunotherapy?. Cell. Mol. Life Sci. 68, 2443–2452 (2011). https://doi.org/10.1007/s00018-011-0706-6
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00018-011-0706-6