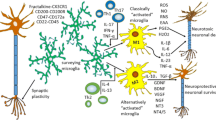
Abstract
Microglial cells contribute to normal function of the central nervous system (CNS). Besides playing a role in the innate immunity, they are also involved in neuronal plasticity and homeostasis of the CNS. While microglial cells get activated and undergo phenotypic changes in different disease contexts, they are far from being the “villains” in the CNS. Mounting evidence indicates that microglial dysfunction can exacerbate the pathogenesis of several diseases in the CNS. Several molecular mechanisms tightly regulate the production of inflammatory and toxic factors released by microglia. These mechanisms involve the interaction with other glial cells and neurons and the fine regulation of signaling and transcription activation pathways. The purpose of this review is to discuss microglia activation and to highlight the molecular pathways that can counteract the detrimental role of microglia in several neurologic diseases. Recent work presented in this review support that the understanding of microglial responses can pave the way to design new therapies for inflammatory diseases of the CNS.

Similar content being viewed by others
References
Schulz C et al (2012) A lineage of myeloid cells independent of Myb and hematopoietic stem cells. Science 336(6077):86–90
Aloisi F, Ria F, Adorini L (2000) Regulation of T-cell responses by CNS antigen-presenting cells: different roles for microglia and astrocytes. Immunol Today 21(3):141–147
Eichhoff G, Brawek B, Garaschuk O (2011) Microglial calcium signal acts as a rapid sensor of single neuron damage in vivo. Biochim Biophys Acta 1813(5):1014–1024
Nimmerjahn A, Kirchhoff F, Helmchen F (2005) Resting microglial cells are highly dynamic surveillants of brain parenchyma in vivo. Science 308(5726):1314–1318
Davalos D et al (2012) Fibrinogen-induced perivascular microglial clustering is required for the development of axonal damage in neuroinflammation. Nat Commun 3:1227
Fuhrmann M et al (2010) Microglial Cx3cr1 knockout prevents neuron loss in a mouse model of Alzheimer’s disease. Nat Neurosci 13(4):411–413
Davalos D et al (2005) ATP mediates rapid microglial response to local brain injury in vivo. Nat Neurosci 8(6):752–758
Ginhoux F et al (2010) Fate mapping analysis reveals that adult microglia derive from primitive macrophages. Science 330(6005):841–845
Wake H et al (2009) Resting microglia directly monitor the functional state of synapses in vivo and determine the fate of ischemic terminals. J Neurosci 29(13):3974–3980
Schafer DP et al (2012) Microglia sculpt postnatal neural circuits in an activity and complement-dependent manner. Neuron 74(4):691–705
Parkhurst CN et al (2013) Microglia promote learning-dependent synapse formation through brain-derived neurotrophic factor. Cell 155(7):1596–1609
Yrjanheikki J et al (1998) Tetracyclines inhibit microglial activation and are neuroprotective in global brain ischemia. Proc Natl Acad Sci USA 95(26):15769–15774
Frank-Cannon TC et al (2009) Does neuroinflammation fan the flame in neurodegenerative diseases? Mol Neurodegener 4:47
Blank T, Prinz M (2013) Microglia as modulators of cognition and neuropsychiatric disorders. Glia 61(1):62–70
Kierdorf K et al (2013) Microglia emerge from erythromyeloid precursors via Pu.1- and Irf8-dependent pathways. Nat Neurosci 16(3):273–280
Hume DA (2006) The mononuclear phagocyte system. Curr Opin Immunol 18(1):49–53
Mildner A et al (2007) Microglia in the adult brain arise from Ly-6ChiCCR2+ monocytes only under defined host conditions. Nat Neurosci 10(12):1544–1553
Simard AR, Rivest S (2004) Bone marrow stem cells have the ability to populate the entire central nervous system into fully differentiated parenchymal microglia. FASEB J 18(9):998–1000
Ajami B et al (2007) Local self-renewal can sustain CNS microglia maintenance and function throughout adult life. Nat Neurosci 10(12):1538–1543
Ransohoff RM (2007) Microgliosis: the questions shape the answers. Nat Neurosci 10(12):1507–1509
Wang Y et al (2012) IL-34 is a tissue-restricted ligand of CSF1R required for the development of Langerhans cells and microglia. Nat Immunol 13(8):753–760
Wirenfeldt M et al (2005) Reactive microgliosis engages distinct responses by microglial subpopulations after minor central nervous system injury. J Neurosci Res 82(4):507–514
Pais TF, Chatterjee S (2005) Brain macrophage activation in murine cerebral malaria precedes accumulation of leukocytes and CD8+ T cell proliferation. J Neuroimmunol 163(1–2):73–83
Logan TT, Villapol S, Symes AJ (2013) TGF-beta superfamily gene expression and induction of the Runx1 transcription factor in adult neurogenic regions after brain injury. PLoS ONE 8(3):e59250
Hanisch UK, Kettenmann H (2007) Microglia: active sensor and versatile effector cells in the normal and pathologic brain. Nat Neurosci 10(11):1387–1394
Codolo G et al (2013) Triggering of inflammasome by aggregated alpha-synuclein, an inflammatory response in synucleinopathies. PLoS ONE 8(1):e55375
Schwartz M et al (2006) Microglial phenotype: is the commitment reversible? Trends Neurosci 29(2):68–74
Martinez FO, Helming L, Gordon S (2009) Alternative activation of macrophages: an immunologic functional perspective. Annu Rev Immunol 27:451–483
Chao CC et al (1992) Activated microglia mediate neuronal cell injury via a nitric oxide mechanism. J Immunol 149(8):2736–2741
Bhat NR et al (1998) Extracellular signal-regulated kinase and p38 subgroups of mitogen-activated protein kinases regulate inducible nitric oxide synthase and tumor necrosis factor-alpha gene expression in endotoxin-stimulated primary glial cultures. J Neurosci 18(5):1633–1641
Takeuchi H et al (2006) Tumor necrosis factor-alpha induces neurotoxicity via glutamate release from hemichannels of activated microglia in an autocrine manner. J Biol Chem 281(30):21362–21368
Meda L et al (1995) Activation of microglial cells by beta-amyloid protein and interferon-gamma. Nature 374(6523):647–650
Pais TF et al (2008) Necrotic neurons enhance microglial neurotoxicity through induction of glutaminase by a MyD88-dependent pathway. J Neuroinflammation 5:43
Maezawa I et al (2011) Amyloid-beta protein oligomer at low nanomolar concentrations activates microglia and induces microglial neurotoxicity. J Biol Chem 286(5):3693–3706
Zhang W et al (2005) Aggregated alpha-synuclein activates microglia: a process leading to disease progression in Parkinson’s disease. FASEB J 19(6):533–542
Block ML, Zecca L, Hong JS (2007) Microglia-mediated neurotoxicity: uncovering the molecular mechanisms. Nat Rev Neurosci 8(1):57–69
Tufekci KU, Genc S, Genc K (2011) The endotoxin-induced neuroinflammation model of Parkinson’s disease. Parkinsons Dis 2011:487450
Freilich RW, Woodbury ME, Ikezu T (2013) Integrated expression profiles of mRNA and miRNA in polarized primary murine microglia. PLoS ONE 8(11):e79416
Zhao W et al (2006) Protective effects of an anti-inflammatory cytokine, interleukin-4, on motoneuron toxicity induced by activated microglia. J Neurochem 99(4):1176–1187
Chao CC, Molitor TW, Hu S (1993) Neuroprotective role of IL-4 against activated microglia. J Immunol 151(3):1473–1481
Shimizu E et al (2008) IL-4-induced selective clearance of oligomeric beta-amyloid peptide(1-42) by rat primary type 2 microglia. J Immunol 181(9):6503–6513
Miron VE et al (2013) M2 microglia and macrophages drive oligodendrocyte differentiation during CNS remyelination. Nat Neurosci 16(9):1211–1218
Ponomarev ED et al (2007) CNS-derived interleukin-4 is essential for the regulation of autoimmune inflammation and induces a state of alternative activation in microglial cells. J Neurosci 27(40):10714–10721
Colton CA et al (2006) Expression profiles for macrophage alternative activation genes in AD and in mouse models of AD. J Neuroinflammation 3:27
Vogel DY et al (2013) Macrophages in inflammatory multiple sclerosis lesions have an intermediate activation status. J Neuroinflammation 10:35
Hanamsagar R, Hanke ML, Kielian T (2012) Toll-like receptor (TLR) and inflammasome actions in the central nervous system. Trends Immunol 33(7):333–342
Klesney-Tait J, Turnbull IR, Colonna M (2006) The TREM receptor family and signal integration. Nat Immunol 7(12):1266–1273
Schmid CD et al (2002) Heterogeneous expression of the triggering receptor expressed on myeloid cells-2 on adult murine microglia. J Neurochem 83(6):1309–1320
Takahashi K, Rochford CD, Neumann H (2005) Clearance of apoptotic neurons without inflammation by microglial triggering receptor expressed on myeloid cells-2. J Exp Med 201(4):647–657
Daws MR et al (2003) Pattern recognition by TREM-2: binding of anionic ligands. J Immunol 171(2):594–599
Stefano L et al (2009) The surface-exposed chaperone, Hsp60, is an agonist of the microglial TREM2 receptor. J Neurochem 110(1):284–294
Chouery E et al (2008) Mutations in TREM2 lead to pure early-onset dementia without bone cysts. Hum Mutat 29(9):E194–E204
Kondo T et al (2002) Heterogeneity of presenile dementia with bone cysts (Nasu-Hakola disease): three genetic forms. Neurology 59(7):1105–1107
Jiang T et al (2013) TREM2 in Alzheimer’s disease. Mol Neurobiol 48(1):180–185
Napoli I, Neumann H (2010) Protective effects of microglia in multiple sclerosis. Exp Neurol 225(1):24–28
Takahashi K et al (2007) TREM2-transduced myeloid precursors mediate nervous tissue debris clearance and facilitate recovery in an animal model of multiple sclerosis. PLoS Med 4(4):e124
Olah M et al (2012) Identification of a microglia phenotype supportive of remyelination. Glia 60(2):306–321
Crocker PR, Paulson JC, Varki A (2007) Siglecs and their roles in the immune system. Nat Rev Immunol 7(4):255–266
Pillai S et al (2012) Siglecs and immune regulation. Annu Rev Immunol 30:357–392
Angata T et al (2002) Cloning and characterization of human Siglec-11. A recently evolved signaling molecule that can interact with SHP-1 and SHP-2 and is expressed by tissue macrophages, including brain microglia. J Biol Chem 277(27):24466–24474
Wang Y, Neumann H (2010) Alleviation of neurotoxicity by microglial human Siglec-11. J Neurosci 30(9):3482–3488
Claude J et al (2013) Microglial CD33-related Siglec-E inhibits neurotoxicity by preventing the phagocytosis-associated oxidative burst. J Neurosci 33(46):18270–18276
Griciuc A et al (2013) Alzheimer’s disease risk gene CD33 inhibits microglial uptake of amyloid beta. Neuron 78(4):631–643
Mihrshahi R, Barclay AN, Brown MH (2009) Essential roles for Dok2 and RasGAP in CD200 receptor-mediated regulation of human myeloid cells. J Immunol 183(8):4879–4886
Wright GJ et al (2000) Lymphoid/neuronal cell surface OX2 glycoprotein recognizes a novel receptor on macrophages implicated in the control of their function. Immunity 13(2):233–242
Lyons A et al (2007) CD200 ligand receptor interaction modulates microglial activation in vivo and in vitro: a role for IL-4. J Neurosci 27(31):8309–8313
Dentesano G et al (2012) Inhibition of CD200R1 expression by C/EBP beta in reactive microglial cells. J Neuroinflammation 9:165
Lyons A et al (2012) Dok2 mediates the CD200Fc attenuation of Abeta-induced changes in glia. J Neuroinflammation 9:107
Hoek RM et al (2000) Down-regulation of the macrophage lineage through interaction with OX2 (CD200). Science 290(5497):1768–1771
Hernangomez M et al (2012) CD200–CD200R1 interaction contributes to neuroprotective effects of anandamide on experimentally induced inflammation. Glia 60(9):1437–1450
Chitnis T et al (2007) Elevated neuronal expression of CD200 protects Wlds mice from inflammation-mediated neurodegeneration. Am J Pathol 170(5):1695–1712
Koning N et al (2009) Distribution of the immune inhibitory molecules CD200 and CD200R in the normal central nervous system and multiple sclerosis lesions suggests neuron–glia and glia–glia interactions. J Neuropathol Exp Neurol 68(2):159–167
Walker DG et al (2009) Decreased expression of CD200 and CD200 receptor in Alzheimer’s disease: a potential mechanism leading to chronic inflammation. Exp Neurol 215(1):5–19
Harrison JK et al (1998) Role for neuronally derived fractalkine in mediating interactions between neurons and CX3CR1-expressing microglia. Proc Natl Acad Sci USA 95(18):10896–10901
Wolf Y et al (2013) Microglia, seen from the CX3CR1 angle. Front Cell Neurosci 7:26
Garton KJ et al (2001) Tumor necrosis factor-alpha-converting enzyme (ADAM17) mediates the cleavage and shedding of fractalkine (CX3CL1). J Biol Chem 276(41):37993–38001
Maciejewski-Lenoir D et al (1999) Characterization of fractalkine in rat brain cells: migratory and activation signals for CX3CR-1-expressing microglia. J Immunol 163(3):1628–1635
Zujovic V et al (2000) Fractalkine modulates TNF-alpha secretion and neurotoxicity induced by microglial activation. Glia 29(4):305–315
Noda M et al (2011) Fractalkine attenuates excito-neurotoxicity via microglial clearance of damaged neurons and antioxidant enzyme heme oxygenase-1 expression. J Biol Chem 286(3):2308–2319
Cardona AE et al (2006) Control of microglial neurotoxicity by the fractalkine receptor. Nat Neurosci 9(7):917–924
Rogers JT et al (2011) CX3CR1 deficiency leads to impairment of hippocampal cognitive function and synaptic plasticity. J Neurosci 31(45):16241–16250
Garcia JA et al (2013) Regulation of adaptive immunity by the fractalkine receptor during autoimmune inflammation. J Immunol 191(3):1063–1072
Zhu W et al (2013) Elevated expression of fractalkine (CX3CL1) and fractalkine receptor (CX3CR1) in the dorsal root ganglia and spinal cord in experimental autoimmune encephalomyelitis: implications in multiple sclerosis-induced neuropathic pain. Biomed Res Int 2013:480702
Wu J et al (2013) Suppression of central chemokine fractalkine receptor signaling alleviates amyloid-induced memory deficiency. Neurobiol Aging 34(12):2843–2852
Lee S et al (2010) CX3CR1 deficiency alters microglial activation and reduces beta-amyloid deposition in two Alzheimer’s disease mouse models. Am J Pathol 177(5):2549–2562
Cho SH et al (2011) CX3CR1 protein signaling modulates microglial activation and protects against plaque-independent cognitive deficits in a mouse model of Alzheimer disease. J Biol Chem 286(37):32713–32722
Saunders AE, Johnson P (2010) Modulation of immune cell signalling by the leukocyte common tyrosine phosphatase, CD45. Cell Signal 22(3):339–348
Ford AL et al (1995) Normal adult ramified microglia separated from other central nervous system macrophages by flow cytometric sorting. Phenotypic differences defined and direct ex vivo antigen presentation to myelin basic protein-reactive CD4+ T cells compared. J Immunol 154(9):4309–4321
Sedgwick JD et al (1991) Isolation and direct characterization of resident microglial cells from the normal and inflamed central nervous system. Proc Natl Acad Sci USA 88(16):7438–7442
Masliah E et al (1991) Immunoreactivity of CD45, a protein phosphotyrosine phosphatase. Alzheimer’s disease. Acta Neuropathol 83(1):12–20
Tan J et al (2000) CD45 opposes beta-amyloid peptide-induced microglial activation via inhibition of p44/42 mitogen-activated protein kinase. J Neurosci 20(20):7587–7594
Zhu Y et al (2008) CD45RB is a novel molecular therapeutic target to inhibit Abeta peptide-induced microglial MAPK activation. PLoS ONE 3(5):e2135
Mott RT et al (2004) Neuronal expression of CD22: novel mechanism for inhibiting microglial proinflammatory cytokine production. Glia 46(4):369–379
Mason LH et al (2006) Regulation of Ly49D/DAP12 signal transduction by Src-family kinases and CD45. J Immunol 176(11):6615–6623
Taylor DL, Diemel LT, Pocock JM (2003) Activation of microglial group III metabotropic glutamate receptors protects neurons against microglial neurotoxicity. J Neurosci 23(6):2150–2160
Lee M, Schwab C, McGeer PL (2011) Astrocytes are GABAergic cells that modulate microglial activity. Glia 59(1):152–165
Lee M (2013) Neurotransmitters and microglial-mediated neuroinflammation. Curr Protein Pept Sci 14(1):21–32
Chan-Palay V, Asan E (1989) Alterations in catecholamine neurons of the locus coeruleus in senile dementia of the Alzheimer type and in Parkinson’s disease with and without dementia and depression. J Comp Neurol 287(3):373–392
Albuquerque EX et al (2009) Mammalian nicotinic acetylcholine receptors: from structure to function. Physiol Rev 89(1):73–120
Zhang L et al (1998) Cholinergic agonists increase intracellular Ca2+ in cultured human microglia. Neurosci Lett 255(1):33–36
Shytle RD et al (2004) Cholinergic modulation of microglial activation by alpha 7 nicotinic receptors. J Neurochem 89(2):337–343
De Simone R et al (2005) Activation of alpha7 nicotinic acetylcholine receptor by nicotine selectively up-regulates cyclooxygenase-2 and prostaglandin E2 in rat microglial cultures. J Neuroinflammation 2(1):4
Mori K et al (2002) Effects of norepinephrine on rat cultured microglial cells that express alpha1, alpha2, beta1 and beta2 adrenergic receptors. Neuropharmacology 43(6):1026–1034
Dello Russo C et al (2004) Inhibition of microglial inflammatory responses by norepinephrine: effects on nitric oxide and interleukin-1beta production. J Neuroinflammation 1(1):9
Waschek JA (2013) VIP and PACAP: neuropeptide modulators of CNS inflammation, injury, and repair. Br J Pharmacol 169(3):512–523
Kim WK et al (2000) Vasoactive intestinal peptide and pituitary adenylyl cyclase-activating polypeptide inhibit tumor necrosis factor-alpha production in injured spinal cord and in activated microglia via a cAMP-dependent pathway. J Neurosci 20(10):3622–3630
Delgado M, Leceta J, Ganea D (2003) Vasoactive intestinal peptide and pituitary adenylate cyclase-activating polypeptide inhibit the production of inflammatory mediators by activated microglia. J Leukoc Biol 73(1):155–164
Delgado M, Varela N, Gonzalez-Rey E (2008) Vasoactive intestinal peptide protects against beta-amyloid-induced neurodegeneration by inhibiting microglia activation at multiple levels. Glia 56(10):1091–1103
Heese K, Hock C, Otten U (1998) Inflammatory signals induce neurotrophin expression in human microglial cells. J Neurochem 70(2):699–707
Neumann H et al (1998) Neurotrophins inhibit major histocompatibility class II inducibility of microglia: involvement of the p75 neurotrophin receptor. Proc Natl Acad Sci USA 95(10):5779–5784
Mizoguchi Y et al (2009) Brain-derived neurotrophic factor induces sustained elevation of intracellular Ca2+ in rodent microglia. J Immunol 183(12):7778–7786
Nakajima K et al (1998) Neurotrophins regulate the function of cultured microglia. Glia 24(3):272–289
Tzeng SF, Huang HY (2003) Downregulation of inducible nitric oxide synthetase by neurotrophin-3 in microglia. J Cell Biochem 90(2):227–233
Yamashita H et al (1994) Formation of hetero-oligomeric complexes of type I and type II receptors for transforming growth factor-beta. J Biol Chem 269(31):20172–20178
Spittau B et al (2013) Endogenous transforming growth factor-beta promotes quiescence of primary microglia in vitro. Glia 61(2):287–300
Qian L et al (2008) Potent anti-inflammatory and neuroprotective effects of TGF-beta1 are mediated through the inhibition of ERK and p47phox-Ser345 phosphorylation and translocation in microglia. J Immunol 181(1):660–668
Chen S et al (2002) TGF-beta1 upregulates CX3CR1 expression and inhibits fractalkine-stimulated signaling in rat microglia. J Neuroimmunol 133(1–2):46–55
Makwana M et al (2007) Endogenous transforming growth factor beta 1 suppresses inflammation and promotes survival in adult CNS. J Neurosci 27(42):11201–11213
Brionne TC et al (2003) Loss of TGF-beta 1 leads to increased neuronal cell death and microgliosis in mouse brain. Neuron 40(6):1133–1145
Hu X et al (2007) Crosstalk among Jak-STAT, Toll-like receptor, and ITAM-dependent pathways in macrophage activation. J Leukoc Biol 82(2):237–243
Natoli G, Ghisletti S, Barozzi I (2011) The genomic landscapes of inflammation. Genes Dev 25(2):101–106
Zhang G et al (2013) Hypothalamic programming of systemic ageing involving IKK-beta. NF-kappaB and GnRH. Nature 497(7448):211–216
Milatovic D et al (2003) Pharmacologic suppression of neuronal oxidative damage and dendritic degeneration following direct activation of glial innate immunity in mouse cerebrum. J Neurochem 87(6):1518–1526
Hayden MS, Ghosh S (2012) NF-kappaB, the first quarter-century: remarkable progress and outstanding questions. Genes Dev 26(3):203–234
Cho IH et al (2008) Role of microglial IKKbeta in kainic acid-induced hippocampal neuronal cell death. Brain 131(Pt 11):3019–3033
Frakes AE et al (2014) Microglia induce motor neuron death via the classical NF-kappaB pathway in amyotrophic lateral sclerosis. Neuron 81(5):1009–1023
Cheret C et al (2008) Neurotoxic activation of microglia is promoted by a nox1-dependent NADPH oxidase. J Neurosci 28(46):12039–12051
Pawate S et al (2004) Redox regulation of glial inflammatory response to lipopolysaccharide and interferongamma. J Neurosci Res 77(4):540–551
Innamorato NG et al (2008) The transcription factor Nrf2 is a therapeutic target against brain inflammation. J Immunol 181(1):680–689
Koh K et al (2011) Transcription factor Nrf2 suppresses LPS-induced hyperactivation of BV-2 microglial cells. J Neuroimmunol 233(1–2):160–167
Ponomarev ED, Veremeyko T, Weiner HL (2013) MicroRNAs are universal regulators of differentiation, activation, and polarization of microglia and macrophages in normal and diseased CNS. Glia 61(1):91–103
Cardoso AL et al (2012) miR-155 modulates microglia-mediated immune response by down-regulating SOCS-1 and promoting cytokine and nitric oxide production. Immunology 135(1):73–88
Parisi C et al (2013) Dysregulated microRNAs in amyotrophic lateral sclerosis microglia modulate genes linked to neuroinflammation. Cell Death Dis 4:e959
Ponomarev ED et al (2011) MicroRNA-124 promotes microglia quiescence and suppresses EAE by deactivating macrophages via the C/EBP-alpha-PU.1 pathway. Nat Med 17(1):64–70
Jenuwein T, Allis CD (2001) Translating the histone code. Science 293(5532):1074–1080
Glozak MA et al (2005) Acetylation and deacetylation of non-histone proteins. Gene 363:15–23
Faraco G et al (2009) Histone deacetylase (HDAC) inhibitors reduce the glial inflammatory response in vitro and in vivo. Neurobiol Dis 36(2):269–279
Giorgini F et al (2008) Histone deacetylase inhibition modulates kynurenine pathway activation in yeast, microglia, and mice expressing a mutant huntingtin fragment. J Biol Chem 283(12):7390–7400
Martinez-Redondo P, Vaquero A (2013) The diversity of histone versus nonhistone sirtuin substrates. Genes Cancer 4(3–4):148–163
Kawahara TL et al (2009) SIRT6 links histone H3 lysine 9 deacetylation to NF-kappaB-dependent gene expression and organismal life span. Cell 136(1):62–74
Yeung F et al (2004) Modulation of NF-kappaB-dependent transcription and cell survival by the SIRT1 deacetylase. EMBO J 23(12):2369–2380
Rothgiesser KM et al (2010) SIRT2 regulates NF-kappaB dependent gene expression through deacetylation of p65 Lys310. J Cell Sci 123(Pt 24):4251–4258
Chen J et al (2005) SIRT1 protects against microglia-dependent amyloid-beta toxicity through inhibiting NF-kappaB signaling. J Biol Chem 280(48):40364–40374
Pandithage R et al (2008) The regulation of SIRT2 function by cyclin-dependent kinases affects cell motility. J Cell Biol 180(5):915–929
Pais TF et al (2013) The NAD-dependent deacetylase sirtuin 2 is a suppressor of microglial activation and brain inflammation. EMBO J 32(19):2603–2616
Beutner C et al (2013) Engineered stem cell-derived microglia as therapeutic vehicle for experimental autoimmune encephalomyelitis. Gene Ther 20(8):797–806
Yasojima K et al (1999) Distribution of cyclooxygenase-1 and cyclooxygenase-2 mRNAs and proteins in human brain and peripheral organs. Brain Res 830(2):226–236
Fan LW et al (2013) Celecoxib attenuates systemic lipopolysaccharide-induced brain inflammation and white matter injury in the neonatal rats. Neuroscience 240:27–38
Lim GP et al (2000) Ibuprofen suppresses plaque pathology and inflammation in a mouse model for Alzheimer’s disease. J Neurosci 20(15):5709–5714
Szekely CA et al (2004) Nonsteroidal anti-inflammatory drugs for the prevention of Alzheimer’s disease: a systematic review. Neuroepidemiology 23(4):159–169
Klegeris A, McGeer PL (2005) Non-steroidal anti-inflammatory drugs (NSAIDs) and other anti-inflammatory agents in the treatment of neurodegenerative disease. Curr Alzheimer Res 2(3):355–365
Aisen PS et al (2003) Effects of rofecoxib or naproxen vs placebo on Alzheimer disease progression: a randomized controlled trial. JAMA 289(21):2819–2826
Reines SA et al (2004) Rofecoxib: no effect on Alzheimer’s disease in a 1-year, randomized, blinded, controlled study. Neurology 62(1):66–71
Yong VW et al (2004) The promise of minocycline in neurology. Lancet Neurol 3(12):744–751
Good ML, Hussey DL (2003) Minocycline: stain devil? Br J Dermatol 149(2):237–239
Aronson AL (1980) Pharmacotherapeutics of the newer tetracyclines. J Am Vet Med Assoc 176(10):1061–1068
Blum D et al (2004) Clinical potential of minocycline for neurodegenerative disorders. Neurobiol Dis 17(3):359–366
Ryan ME, Greenwald RA, Golub LM (1996) Potential of tetracyclines to modify cartilage breakdown in osteoarthritis. Curr Opin Rheumatol 8(3):238–247
Lin S et al (2001) Minocycline blocks nitric oxide-induced neurotoxicity by inhibition p38 MAP kinase in rat cerebellar granule neurons. Neurosci Lett 315(1–2):61–64
Pi R et al (2004) Minocycline prevents glutamate-induced apoptosis of cerebellar granule neurons by differential regulation of p38 and Akt pathways. J Neurochem 91(5):1219–1230
Wu DC et al (2002) Blockade of microglial activation is neuroprotective in the 1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine mouse model of Parkinson disease. J Neurosci 22(5):1763–1771
Yrjanheikki J et al (1999) A tetracycline derivative, minocycline, reduces inflammation and protects against focal cerebral ischemia with a wide therapeutic window. Proc Natl Acad Sci USA 96(23):13496–13500
Ferretti MT et al (2012) Minocycline corrects early, pre-plaque neuroinflammation and inhibits BACE-1 in a transgenic model of Alzheimer’s disease-like amyloid pathology. J Neuroinflammation 9:62
Biscaro B et al (2012) Inhibition of microglial activation protects hippocampal neurogenesis and improves cognitive deficits in a transgenic mouse model for Alzheimer’s disease. Neurodegener Dis 9(4):187–198
Seabrook TJ et al (2006) Minocycline affects microglia activation, Abeta deposition, and behavior in APP-tg mice. Glia 53(7):776–782
Popovic N et al (2002) Inhibition of autoimmune encephalomyelitis by a tetracycline. Ann Neurol 51(2):215–223
Nikodemova M et al (2007) Minocycline down-regulates MHC II expression in microglia and macrophages through inhibition of IRF-1 and protein kinase C (PKC)alpha/betaII. J Biol Chem 282(20):15208–15216
Plane JM et al (2010) Prospects for minocycline neuroprotection. Arch Neurol 67(12):1442–1448
Metz LM et al (2004) Minocycline reduces gadolinium-enhancing magnetic resonance imaging lesions in multiple sclerosis. Ann Neurol 55(5):756
Zabad RK et al (2007) The clinical response to minocycline in multiple sclerosis is accompanied by beneficial immune changes: a pilot study. Mult Scler 13(4):517–526
Zhang Y et al (2008) Pilot study of minocycline in relapsing–remitting multiple sclerosis. Can J Neurol Sci 35(2):185–191
Liu Y et al (2006) Suppression of microglial inflammatory activity by myelin phagocytosis: role of p47-PHOX-mediated generation of reactive oxygen species. J Neurosci 26(50):12904–12913
Murugaiyan G et al (2011) Silencing microRNA-155 ameliorates experimental autoimmune encephalomyelitis. J Immunol 187(5):2213–2221
Akerblom M et al (2013) Visualization and genetic modification of resident brain microglia using lentiviral vectors regulated by microRNA-9. Nat Commun 4:1770
Tabas I, Glass CK (2013) Anti-inflammatory therapy in chronic disease: challenges and opportunities. Science 339(6116):166–172
Liu T et al (1994) Tumor necrosis factor-alpha expression in ischemic neurons. Stroke 25(7):1481–1488
Rieckmann P et al (1995) Tumor necrosis factor-alpha messenger RNA expression in patients with relapsing–remitting multiple sclerosis is associated with disease activity. Ann Neurol 37(1):82–88
Alvarez A et al (2007) Serum TNF-alpha levels are increased and correlate negatively with free IGF-I in Alzheimer disease. Neurobiol Aging 28(4):533–536
McCoy MK, Tansey MG (2008) TNF signaling inhibition in the CNS: implications for normal brain function and neurodegenerative disease. J Neuroinflammation 5:45
He P et al (2007) Deletion of tumor necrosis factor death receptor inhibits amyloid beta generation and prevents learning and memory deficits in Alzheimer’s mice. J Cell Biol 178(5):829–841
McCoy MK et al (2006) Blocking soluble tumor necrosis factor signaling with dominant-negative tumor necrosis factor inhibitor attenuates loss of dopaminergic neurons in models of Parkinson’s disease. J Neurosci 26(37):9365–9375
McCoy MK et al (2008) Intranigral lentiviral delivery of dominant-negative TNF attenuates neurodegeneration and behavioral deficits in hemiparkinsonian rats. Mol Ther 16(9):1572–1579
Cox FF et al (2012) CD200 fusion protein decreases microglial activation in the hippocampus of aged rats. Brain Behav Immun 26(5):789–796
Minami SS et al (2012) Selective targeting of microglia by quantum dots. J Neuroinflammation 9:22
Priller J et al (2001) Targeting gene-modified hematopoietic cells to the central nervous system: use of green fluorescent protein uncovers microglial engraftment. Nat Med 7(12):1356–1361
Derecki NC et al (2012) Wild-type microglia arrest pathology in a mouse model of Rett syndrome. Nature 484(7392):105–109
Sawada M et al (1998) Brain-specific gene expression by immortalized microglial cell-mediated gene transfer in the mammalian brain. FEBS Lett 433(1–2):37–40
Acknowledgments
We are thankful to Oldriska Chutna, Pedro Antas and Maria Salomé Gomes for critical editing and reading of the manuscript.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Fernandes, A., Miller-Fleming, L. & Pais, T.F. Microglia and inflammation: conspiracy, controversy or control?. Cell. Mol. Life Sci. 71, 3969–3985 (2014). https://doi.org/10.1007/s00018-014-1670-8
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00018-014-1670-8