Abstract
Physical exercise induces cell proliferation in the adult hippocampus in rodents. Serotonin (5-HT) and angiotensin (Ang) II are important mediators of the pro-mitotic effect of physical activity. Here, we examine precursor cells in the adult brain of mice lacking angiotensin-converting enzyme (ACE) 2, and explore the effect of an acute running stimulus on neurogenesis. ACE2 metabolizes Ang II to Ang-(1–7) and is essential for the intestinal uptake of tryptophan (Trp), the 5-HT precursor. In ACE2-deficient mice, we observed a decrease in brain 5-HT levels and no increase in the number of BrdU-positive cells following exercise. Targeting the Ang II/AT1 axis by blocking the receptor, or experimentally increasing Trp/5-HT levels in the brain of ACE2-deficient mice, did not rescue the running-induced effect. Furthermore, mice lacking the Ang-(1–7) receptor, Mas, presented a normal neurogenic response to exercise. Our results identify ACE2 as a novel factor required for exercise-dependent modulation of adult neurogenesis and essential for 5-HT metabolism.
Similar content being viewed by others
Introduction
In the adult mammalian brain, continuous generation of new neurons takes place in a few spatially restricted regions, where neural stem cells retain fate plasticity [1] and respond to environmental stimuli [2, 3]. One strong external effector is voluntary wheel running that induces progenitor cell proliferation in the adult dentate gyrus (DG) of the hippocampus [3, 4]. We have recently established serotonin (5-HT) as an important mediator of the pro-mitotic effect of physical exercise [5]. Besides 5-HT, other soluble factors released by surrounding niches such as the vasculature (e.g., IGF-1, VEGF, neuropeptide orexin-A [6,7,8]) participate in modulation of the proliferative phase in adult neurogenesis and are also abundant in blood circulation.
The renin-angiotensin system (RAS) is the main humoral system of the body that regulates cardiovascular homeostasis. Angiotensin (Ang)-converting enzyme 2 (ACE2) is a principal regulatory enzyme of the RAS that catalyzes the conversion of Ang II to Ang-(1–7) [9]. In this role, ACE2 controls the transition from Ang II signaling, which is vasoconstrictive and pro-inflammatory to that of Ang-(1–7), which is vasodilatory and anti-inflammatory. Ang II exerts its deleterious effects by binding to the Ang II receptor type 1 (AT1), and selective AT1 antagonists are used clinically to lower blood pressure in hypertensive patients. The G-protein-coupled receptor Mas has been identified as a functional receptor of Ang-(1–7) [10]. Together with the Ang II receptor type 2 (AT2), the ACE2/Ang-(1–7)/Mas axis represents the “protective arm” of the RAS. Besides the well-described role in systemic cardiovascular and metabolic regulations [11, 12], ACE2 and Mas may be also involved in central effects mediated by a local RAS in the brain [13, 14]. Both components are expressed in the adult brain with particular density in parts of the hippocampus where adult neurogenesis occurs [15,16,17].
Recent studies attribute RAS-independent functions to ACE2 as it serves as SARS coronavirus receptor [18], and as it interacts with the neutral amino acid (AA) transporter B0AT1 via its collectrin-like domain [19, 20]. Specifically, studies suggest a role of ACE2 in the absorption of dietary large neutral AA in the gut, since lack of ACE2 led to a 70% reduction in l-tryptophan (Trp) plasma levels [21, 22]. Trp is an essential AA which crosses the blood brain barrier (BBB) via the large neutral AA transporter 1 (LAT1) [23]. Given that Trp is the precursor of 5-HT, ACE2 could indirectly modulate brain 5-HT levels that are known to affect adult neurogenesis [24].
In our study, we verified that 5-HT synthesis is dependent on ACE2 and investigated the role of the main axes of the RAS (Ang II/AT1 and Ang-(1–7)/Mas) in adult neurogenesis. We used mice with genetic deletion of ACE2 and Mas to examine the effect of voluntary wheel running on cell proliferation in the adult hippocampus. We observed that ACE2 deletion results in an uncoupling of physical activity and neurogenesis. Experimentally elevated Trp levels and blockade of AT1 could not reverse this effect. Loss of Mas instead left the system of running-induced cell proliferation intact. These data indicate that ACE2 is required for exercise-dependent neurogenesis and is an important new modulator of 5-HT metabolism.
Aim of the study There are gaps in our understanding on how brain and circulatory factors co-signal to regulate behavior. In our study, we aim to discover a pathway that links running and brain plasticity that could be exploited as target for modulation of neuron replacement or to augment the effect of physical activity.
Materials and methods
Animals and treatment
Ace2-deficient and Mas-deficient female mice (8–12 weeks old, [25, 26]) were used in this study with both strains back-crossed to C57BL/6 for more than seven generations. Age and background-matched wild-type (WT) female mice were used in all experiments. Animals were randomized and divided into groups for “baseline” conditions and “running” conditions and held for 6–10 days with a 12 h light/dark cycle and ad libitum access to food and water. Mice in running conditions were single housed with unlimited access to a running wheel for 6 days; running distances were monitored daily with computers. We have used single housing that allows monitoring of the running performance per individual mouse. No isolation effects were observed as has been described previously for different mouse strains [5, 27]. In an additional experiment, a cohort of 7-week-old WT mice was used with one group having restricted access to a running wheel per night for 6 days (WT, WTRUN, WTRUN1/2). To analyze cell proliferation, animals received three intraperitoneal injections (i.p.) of bromodeoxyuridine (BrdU, SIGMA-Aldrich, Germany; 50 mg/kg body weight dissolved in 0.9% NaCl) on day 6–6 h apart–and were killed 24 h after the first injection.
The selective AT1 antagonist TEL (telmisartan, 5139, TOCRIS, Wiesbaden-Nordenstadt, Germany) was administered to WT and ACE2-deficient mice daily (i.p.; 10 mg/kg body weight; stock diluted in 1 N NaOH and the pH adjusted to 9.5 by HCl) 2 days before and during 6 days of running. Control running groups received 0.9% NaCl injections. Likewise, WT baseline animals received eight injections of either TEL or vehicle. For the Trp rescue experiment, standard chow (0.25% sodium) was supplemented with glycyl-l-tryptophan (Gly-Trp, BACHEM, Germany; 10 mg/g dry food, corresponding to a dose of 1.5–2 g/kg BW per day) and given 3 days before and during running. All experiments were performed according to national and institutional guidelines and were approved by the appropriate authority, Landesamt fuer Arbeitsschutz, Gesundheitsschutz und technische Sicherheit (LAGeSo) of the State of Berlin, approval number TVV G0300/13.
HPLC analysis
Another set of animals was used for high-performance liquid chromatography (HPLC) analysis. For baseline analysis of Trp, 5-HT and 5-HIAA levels, n = 5 per genotype, and for the Trp rescue experiment n = 6 animals per genotype were used. Animals were deeply anesthetized with ketamine/xylazine (100 mg/kg ketamine, 10 mg/kg xylazine, i.p.) and 300 µl blood was collected by intracardiac puncture with a syringe containing 100 µl of 5000 U/ml heparin (0064N01 Braun, Germany). Heparinized blood was immediately mixed with 15 µl of 10 μl perchloric acid (70%) and 5 μl ascorbic acid (10 mg/ml), thoroughly vortexed for 30 s and subsequently centrifuged for 30 min at 20,000×g and 4 °C. The supernatant was kept at − 80 °C until HPLC analysis. For brain monoamine analysis, mice were subsequently perfused transcardially with 0.9% NaCl. Brains were removed, weighed, and snap-frozen on dry ice. Frozen brain tissue was homogenized in lysis buffer containing 10 µM ascorbic acid and 1.8% perchloric acid, and deproteinized by centrifugation for 30 min at 20,000×g and 4 °C.
Blood and tissue levels of Trp, 5-HT, and 5-HIAA were analyzed using high sensitive HPLC with fluorometric detection (Shimadzu, Tokyo, Japan) [28] as described previously [29] and normalized to wet tissue weight for statistical analysis.
Immunohistochemistry and quantification
Mice were deeply anesthetized and perfused transcardially with 0.9% NaCl, followed by 4% paraformaldehyde (PFA) in 0.1 M phosphate buffer. Brains were removed from the skulls, postfixed in 4% PFA at 4 °C overnight, and transferred to 30% sucrose. Sequential 40 µm coronal sections were cut and cryoprotected. For BrdU staining, DNA was denatured in 2 N HCl for 20 min at 37 °C. Sections were rinsed in 0.1 M borate buffer and washed in Tris-buffered saline (TBS). Sections were stained free floating with all antibodies diluted in TBS containing 3% donkey serum and 0.1% Triton X-100. Primary antibodies were applied in the following concentrations: anti-BrdU (rat, 1:500; Biozol/AbD serotec), anti-doublecortin (DCX; goat, 1:250; Santa Cruz Biotechnology), anti-GFAP (mouse, 1:1000; SIGMA), anti-Sox2 (goat, 1:1000; Santa Cruz Biotechnology). For immunofluorescence, Alexa488-conjugated, Cy3-conjugated, or Alexa647-conjugated secondary antibodies (Jackson ImmunoResearch Laboratories; Invitrogen) were used. IHC for BrdU followed the peroxidase method with biotinylated secondary antibody (Donkey Anti-Rat Biotin, 1:500; Jackson ImmunoResearch Laboratories), ABC Elite reagent (Vector Laboratories), and di-aminobenzidine (DAB; Vector Laboratories) as chromogen.
For quantification, one-in-six series of sections of each brain were BrdU stained for light microscopy (peroxidase method), and immunoreactive cells were counted throughout the rostro-caudal extent of the DG and multiplied by six to obtain the absolute numbers per DG. One-in-twelve series of sections were labeled for multiple immunofluorescence staining as described above for phenotypic analysis. Fifty to 100 randomly selected cells per animal were evaluated for three-dimensional colocalization using a Leica TCS SP5 (Leica, Germany) confocal microscope; the percentage was extrapolated to absolute BrdU numbers.
Statistical analysis
Statistical significance was evaluated by one-way analysis of variance (ANOVA) followed by Tukey’s or Dunnett’s post hoc tests in cases where a significant F statistics was obtained. For individual comparisons, an unpaired two-tailed Student’s t test was used. All values are expressed as mean ± standard error of the mean. p values of ≤ 0.05 were considered to be statistically significant.
Results
ACE2 is required for exercise-induced progenitor cell proliferation
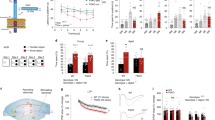
We used ACE2-deficient (ACE2) mice to quantify baseline proliferation in the DG and the number of precursor cells following acute voluntary wheel running (Fig. 1a). Young-adult ACE2-deficient mice display a lower body weight compared with WT mice (8 weeks old, WT 19.8 ± 0.3 g vs. ACE2 17.3 ± 0.4 g, p < 0.0001). The number of proliferating cells in ACE2-deficient mice 24 h after the first BrdU injection did not differ compared with WT mice (WT 1337 ± 99 cells vs. ACE2 1301 ± 97 cells; Fig. 1b). These data show that ACE2 is not required for baseline proliferation of precursor cells. We next tested the neurogenic response to physical exercise. While WT animals revealed the typical robust increase in the number of BrdU-positive (BrdU+) cells in the DG (baseline 1337 ± 99 cells vs. running 3107 ± 159 cells, p < 0.001), 1 week of physical exercise did not increase cell proliferation in ACE2-deficient mice (Fig. 1b). However, we observed that ACE2-deficient mice ran shorter distances during voluntary wheel running for 6 days (WT 59.2 ± 7.5 km vs. ACE2 43.1 ± 4.6 km, p = 0.0012; Fig. 1c). No difference was seen within both genotypes in the mean distances traveled per night and between day 1 and day 6 (d1/d6, WT 10.8 ± 0.5/9.3 ± 1.1 km, ACE2 7.2 ± 0.8/6.2 ± 1.1 km), or in body weight gain between WT (1.14 ± 0.2 g) and ACE2-deficient mice (1.13 ± 0.4 g) over the time course of the experiments. To ensure that the observed reduced running can provoke a neurogenic response, running wheels for an additional WT group were blocked for half of the night to mimic the activity of ACE2-deficient animals. The set of WT mice used for this experiment (WT vs. WTRUN vs. WTRUN1/2) matched the body weight of ACE2-deficient mice (7 weeks old, 17.8 ± 0.4 g). Physical exercise led to a strong increase in BrdU numbers in both running groups (WT 1557 ± 80 cells vs. WTRUN 2800 ± 169 cells, p = 0.0022; vs. WTRUN1/2 3094 ± 201 cells, p = 0.0018; Fig. 1d), although mice with blocked wheels were running significantly less for 6 days (WTRUN 50.3 ± 3.5 km vs. WTRUN1/2 19.9 ± 3.9 km, p = 0.0012; Fig. 1e). With this data we confirm that the amount of running of ACE2-deficient mice should be enough to provoke an increase in cell proliferation.
ACE2-deficient mice (ACE2) reveal no running-induced neurogenesis. a The number of proliferating cells in running animals and sedentary controls (baseline) was quantified on day 7, 24 h after the first of three i.p. BrdU injections. b At baseline, no changes in the absolute number of BrdU-positive cells were observed between WT and ACE2. However, voluntary wheel running robustly increased proliferation in WT with no effect on ACE2. One-way ANOVA F(3,19) = 51.36, p < 0.0001; followed by Tukey’s post hoc test, ***p < 0.001 indicates statistical significance relatively to sedentary WT control, ###p < 0.001 between WT and ACE2 for the same condition; data are presented as mean ± SEM. c ACE2 ran significantly shorter distances than WT. Unpaired Student’s t test, ##p < 0.01. d Running significantly shorter distances than their WT controls (Unpaired Student’s t test, **p < 0.01, e), WTRUN1/2 mice revealed the same robust increase in BrdU-positive cells. One-way ANOVA F(2.9) = 28.09, p = 0.0001; followed by Tukey’s post hoc test, **p < 0.01 indicates statistical significance relatively to sedentary WT control. f Representative images of peroxidase staining of BrdU-positive cells in the subgranular zone (SGZ) for baseline and running in WT and ACE2; scale bar 100 µm. g Running robustly induced the number of cells co-expressing either BrdU/Sox2 (type-2a) or BrdU/DCX (type-2b/3) in the adult dentate gyrus of WT. No increase in these cell types occurred in ACE2. The number of BrdU/GFAP type-1 cells did not change between conditions and genotypes. One-way ANOVA Ftype-1(3,12) = 0.686, p = 0.5173, Ftype-2a(3,13) = 30.10, p < 0.0001, Ftype-2b/3(3,13) = 0.686, p < 0.0001; followed by Tukey’s post hoc test, ***p < 0.001 indicates statistical significance relatively to sedentary controls of the same genotype, ###p < 0.001 between WT and ACE2 for the same condition. The table represents BrdU phenotype distribution in percentage; data are presented as mean ± (SEM), **p < 0.01, ***p < 0.001 relatively to sedentary WT control, ###p < 0.001 between WT and ACE2 for running conditions
Quantitative assessment of the phenotype of newly generated cells for the first experiment (Fig. 1b, f) revealed a characteristic increase in the number of Sox2+ type-2a, and doublecortin (DCX)+ type-2b/3 cells for WT mice after running (Fig. 1g), with Sox2 labeling precursor cells of the neural lineage and DCX as transient marker for immature neurons in the adult DG [30]. The number of these cell types remained constant in ACE2-deficient mice after physical exercise. No changes have been observed after running in the number of BrdU/GFAP+ type-1 cells (morphology-wise radial glia-like stem cells) in both ACE2-deficient and WT animals. At baseline, no difference in phenotype expression was seen between genotypes (Fig. 1g). Cell distribution (Fig. 1g) was unchanged between genotypes and conditions with one exception: The percentage of BrdU/DCX+ cells in WT mice after running was significantly decreased, accompanied by a slight increase in the Sox2+ cell population. Together, these data demonstrate that running-induced cell proliferation is absent in mice that lack ACE2.
Targeting the Ang II/AT1 axis by blocking AT1 does not diminish the ACE2 repression effect
ACE2 deficiency shifts the balance of the two peptides, Ang II and Ang-(1–7), in favor of Ang II. The reduction in Ang II degradation in ACE2-deficient mice [14] might contribute to the suppression of running-induced cell proliferation. To investigate this hypothesis, we blocked AT1 signaling as an approach to reverse the phenotype. Mice were daily injected with telmisartan (TEL; Fig. 2a), a selective AT1 antagonist, which crosses the BBB [31]. Treatment with the antagonist for 1 week did not affect the number of BrdU+ cells in WT animals at baseline (Fig. 2b), or the running performance within genotypes (Fig. 2c). Daily TEL treatment plus running significantly increased the number of proliferating cells in WT mice (WTTEL 2253 ± 106 cells vs. WTRUN+TEL 3259 ± 302 cells, p = 0.0080; Fig. 2b, d), although to a moderate extent when compared with WT control groups (WT 2055 ± 188 cells vs. WTRUN 4924 ± 584 cells, p = 0.0009; Fig. 2b, d). Nevertheless, neither running nor running plus TEL treatment affected the number of BrdU+ cells in ACE2-deficient mice (ACE2RUN 2636 ± 780 cells vs. ACE2RUN+TEL 2296 ± 239 cells, p = 0.6585; Fig. 2b). These experiments show that pharmacological targeting AT1 does not restore the pro-mitotic effect of running in ACE2-deficient mice.
Inhibition of AT1 attenuates the pro-mitotic running effect in WT, but does not affect ACE2-deficient mice (ACE2). a Telmisartan (TEL) was injected once daily into running animals and sedentary WT controls (baseline) 2 days before, and during 6 days of running. The number of proliferating cells was quantified at day 7, 24 h after the first of three i.p. BrdU injections. b At baseline, no difference in the number of BrdU-positive cells was observed between saline and TEL-treated WT groups. Voluntary wheel running induced an increase in BrdU numbers in WT with an attenuated increase following TEL treatment. In ACE2, no changes in cell proliferation were observed following exercise, and TEL administration plus running. One-way ANOVA, saline treatment F(2,13) = 9.394, p = 0.0024; One-way ANOVA, TEL treatment F(2,13) = 6.601, p = 0.0105; followed by Dunnett’s post hoc tests, *p < 0.05, **p < 0.01 indicates statistical significance relatively to WT baseline groups, and #p < 0.05 between genotypes of the same condition; WTRUN vs. WTRUN+TEL Student’s t test $p = 0.0416; data are presented as mean ± SEM. c TEL did not alter running distances in mice of both genotypes with ACE2 generally running less kilometers. One-way ANOVA F(3,14) = 7.700, p = 0.0028. d Images of peroxidase-stained BrdU-positive cells in the subgranular zone of the dentate gyrus for WT baseline, running, and running plus TEL treatment. Scale bar 100 µm
Ang-(1–7)/MAS axis is not involved in the neurogenic response to running
ACE2 is the main enzyme responsible for the generation of Ang-(1–7); lack of ACE2 mimics inactivation of Ang-(1–7) signaling. To evaluate Ang-(1–7) effects in the neurogenic actions of ACE2, we quantified running-induced cell proliferation in the DG of mice deficient in the Ang-(1–7) receptor, Mas (Fig. 3a). At baseline, 8 weeks old Mas-deficient mice (Mas) exhibited a similar number of BrdU+ cells 24 h following the first injection when compared with WT (WT 1537 ± 87 cells vs. Mas 1612 ± 125 cells; Fig. 3b). Voluntary wheel running for 6 days led to a robust pro-mitotic effect in both genotypes compared with baseline (WT 2909 ± 160 cells, p < 0.001, and Mas 2911 ± 189 cells, p < 0.001; Fig. 3b). Running distances observed in WT and Mas animals did not differ between genotypes (WT 55.6 ± 2.1 km vs. Mas 51.5 ± 4.8 km; Fig. 3c). Examination of the phenotypes of newly generated cells revealed no genotype differences in the distribution of BrdU/GFAP+ type-1 (WT 6.8 ± 0.8% vs. Mas 6.4 ± 1.7%), Sox2+ type-2a (WT 29.6 ± 2.5% vs. Mas 35.3 ± 3.0%) and DCX+ type-2b/3 cells (WT 32.2 ± 3.1% vs. Mas 34.0 ± 2.0%) for Mas-deficient and WT mice (representative images; Fig. 3d, e). These data demonstrate that Mas-deficiency has no obvious effect on cell proliferation in the adult DG at baseline and following running. The results show that impaired Ang-(1–7) signaling is not responsible for the lack of running-induced neurogenesis in ACE2-deficient mice.
Mas-deficient mice (Mas) reveal normal levels of cell proliferation in the adult dentate gyrus (DG). a The number of proliferating cells in running animals and sedentary controls (baseline) was quantified on day 7, 24 h after the first of three i.p. BrdU injections. b No difference in the number of BrdU-positive cells was observed at baseline or following running in Mas compared with WT. One-way ANOVA, F(3,19) = 31.65, p < 0.0001, followed by Tukey’s post hoc test, ***p < 0.001 relatively to sedentary control of the same genotype; data are presented as mean ± SEM. c No difference was observed in running distances between genotypes. d, e Representative confocal microscopy images of Sox2/BrdU/GFAP (d) and DCX/BrdU co-expression (e, arrows) in the adult DG of Mas mice; scale bar 100 µm
ACE2 deficiency results in decreased Trp and 5-HT levels in the adult brain
Given that ACE2 also plays a role in the absorption of Trp in the gut, decreased Trp plasma levels in ACE2-deficient mice may result in reduced 5-HT levels in the brain; that in turn could affect running-induced neurogenesis [5]. We therefore evaluated the levels of Trp and 5-HT in the blood and brain of adult ACE2-deficient and WT mice using HPLC analysis. Our results confirm previously described observations [22] of a strong decrease of Trp levels in the blood of ACE2-deficient mice (75% decrease, Fig. 4a). Our data show that this consequently results in significantly lower blood 5-HT levels (WT 2491 ± 250 vs. ACE2 1287 ± 55 pg/mg; p = 0.0015; Fig. 4b). Furthermore, Trp levels in the adult brain of ACE2-deficient mice were also significantly reduced by 64% (WT 2629 ± 193 vs. ACE2 948 ± 79 pg/mg, p < 0.001, Fig. 4c) leading to a 32% decrease in brain 5-HT levels (WT 678 ± 13 vs. ACE2 464 ± 6 pg/mg, p < 0.001; Fig. 4d). Together with decreased levels of the 5-HT degradation product, 5-hydroxyindoleacetic acid (5-HIAA; 71% decrease, WT 468 ± 21 vs. ACE2 136 ± 5.2 pg/mg; p < 0.001, Fig. 4e), this indicates that 5-HT synthesis rate in the brain is drastically downregulated in the absence of ACE2.
Decreased baseline Trp and 5-HT levels in ACE2-deficient mice (ACE2). a–e Levels of Trp (a, c), 5-HT (b, d), and 5-HIAA, the degradation product of 5-HT (e), were significantly decreased in the blood (a, b) and brain (c–e) of ACE2 compared with WT. Unpaired Student’s t test, **p < 0.01, ***p < 0.001 indicate statistical significance relatively to WT; data are presented as mean ± SEM
Normalization of 5-HT levels in ACE2-deficient mice does not restore the pro-mitotic effect
We last tested whether an enforced restoration in Trp levels will lead to a normalization of 5-HT levels in the adult brain of ACE2-deficient mice and thus will rescue increased cell proliferation after physical exercise (Fig. 5a). To restore Trp levels, animals received a diet supplemented with the dipeptide Gly-Trp, which is transported through the gut by ACE2-independent mechanisms and effectively used in previous studies [21]. One week of Gly-Trp-enriched diet (Trp diet) let Trp and 5-HT levels in the blood and brain of ACE2-deficient mice increase. In the brain, the Trp levels increased up to 150% of WT (p < 0.001) and normalized 5-HT to the WT level (Fig. 5b). Running under Trp diet also elevated Trp levels in the brain of ACE2-deficient mice to WT levels (Fig. 5c). No significant difference in 5-HT levels was observed between genotypes following physical exercise and under Trp diet. To test whether Trp diet affects running-induced cell proliferation, the same experimental design was used with Trp diet fed 3 days before and during physical exercise to ACE2-deficient and WT animals. Body weight was not affected by Trp diet, and remained lower in ACE2-deficient mice (12 weeks old, WT 22.8 ± 0.3 g vs. ACE2 20.4 ± 0.3 g, p = 0.006). Surprisingly, WT mice fed Trp diet were running a little less than WT controls, while no difference in running performance was observed for ACE2-deficient mice (WT 53.8 ± 4.5 km vs. WTTrp 39.0 ± 4.4 km, p = 0.05; ACE2 26.7 ± 5.7 km vs. ACE2Trp 20.5 ± 3.5 km, p = 0.375; Fig. 5d). Trp diet did not affect BrdU+ cell numbers in non-runners of either genotype (Fig. 5e). Following exercise, the number of BrdU+ cells robustly increased in WT mice fed standard diet (> threefold, baseline 1226 ± 54 cells vs. running 3819 ± 366 cells, p < 0.001) with a significant > twofold increase observed under Trp diet (WTTrp 1143 ± 78 cells vs. WTRun+Trp 2401 ± 421 cells, p = 0.0036; Fig. 5e). Student’s t test reveals significance between WTRUN vs. WTRUN+Trp, p = 0.038. No increase in the number of BrdU+ cells following running plus Trp diet was observed in ACE2-deficient mice (Fig. 5e). The results indicate that lowered Trp and 5-HT levels in the adult brain of ACE2-deficient mice do not contribute to the lack of the pro-mitotic effect of running.
Experimentally increased Trp levels do not rescue the pro-mitotic running effect in ACE2-deficient mice (ACE2). a Trp diet was administered to sedentary controls (baseline) and running animals 3 days before and during 6 days running. The number of proliferating cells was quantified at day 7, 24 h after the first of three i.p. BrdU injections. b, c When fed Trp diet, Trp levels in the brain of ACE2 increased up to 150% of untreated WT with 5-HT levels reaching baseline WT levels (b). One-way ANOVA FTrp(2,12) = 28.18, p < 0.0001, and F5-HT(2,12) = 41.79, p < 0.0001; followed by Dunnett’s post hoc tests, ##p < 0.01, ###p < 0.001 to WT. Running plus Trp diet (c) also elevated Trp levels in the brain of ACE2. No significant difference in 5-HT levels was observed following physical exercise under Trp diet. One-way ANOVA FTrp(3,17) = 3.66, p = 0.0333; Student’s t test #p = 0.0414 between WT and ACE2, *p = 0.0476 between WT groups, and *p = 0.0372 within ACE2. d When fed Trp diet, WT ran less than their standard diet controls with ACE2 overall running less. One-way ANOVA F(3,16) = 11.59, p < 0.0001; Student’s t test t(7) = 2.342, *p = 0.05 between WT and WTTrp. e Trp diet did not affect BrdU numbers at baseline. Voluntary wheel running induced an increase in the number of progenitor cells in WT with an attenuated increase following Trp diet. In ACE2, the number of BrdU-positive cells did not increase with increased Trp levels. One-way ANOVA for standard diet F(3,15) = 35.14, ***p < 0.001; One-way ANOVA for Trp diet F(3,21) = 7.936, p = 0.0010; followed by Tukey’s post hoc test, ***p < 0.001 relatively to WT baselines, and #p < 0.05, ###p < 0.001 between genotypes of the same condition; Students t test WTRUN vs. WTRUN+Trp $p = 0.038; data are presented as mean ± SEM
Discussion
We discovered that permanent depletion of ACE2 obliterates the effect of the acute running stimulus on cell proliferation in the DG. Our data demonstrate that lack of ACE2 leads to a significant decrease in 5-HT levels in the blood and adult brain of mice defining ACE2 as a new modulator in 5-HT synthesis. 5-HT is necessary for a fast neurogenic response in the hippocampus to changes in physical activity [5]. However, experimentally enhanced Trp and 5-HT concentrations in the brain did not rescue the running-induced effect in ACE2-deficient mice. Targeting the Ang II/AT1 axis by blocking the receptor did also not reverse the findings, and deletion of the receptor for Ang-(1–7) did not phenocopy ACE2 ablation. Our data highlight a novel central nervous system role for ACE2 in that it affects cell proliferation in the DG following physical exercise by a yet unresolved mechanism not involving 5-HT and angiotensins.
We establish that the acute running stimulus requires ACE2 activity. Lack of ACE2 led to changes in running performance, and no pro-mitotic effect in the hippocampus was observed. We ruled out that ACE2-deficient mice lack the capacity for exercise due to reduced body weight. In our previous study, Tph2−/− mice (which lack the brain 5-HT-synthesizing enzyme [32]) displayed growth retardation without affecting running performance [5]; thus, lower body weight of ACE2-deficient mice per se is unlikely to be the reason for reduced physical activity. Furthermore, the pro-mitotic effect of running does not only depend on the distance traveled but the amount of activity/duration [33]. Although running less, ACE2-deficient mice in our study ran continuously every night. As shown for WT mice, long-term voluntary wheel running leads to alterations in muscle strength (e.g., increased muscle mass and skeletal muscle hypertrophy) that was abolished in ACE2-deficient mice [34]. Skeletal muscle releases cathepsin B during long-term treadmill running in monkeys that might affect brain function [35]. Whether acute running in our study leads to differential changes in skeletal muscle between the genotypes and to an altered release of factors influencing adult neurogenesis needs to be determined. However, we did not observe changes in motor behavior per se that might lead to slower running activity. Furthermore, ACE2 deficiency is accompanied by altered glucose homeostasis [36] which may also be implicated in the impaired running-induced neurogenesis. However, Mas-deficient mice show comparable disturbances in cardiac and skeletal muscle physiology [37, 38] and metabolic functions [39] to ACE2-deficient animals and still exhibit normal effects of exercise on cell proliferation in the hippocampus (Fig. 3). Together with our data on increased cell proliferation after mimicking shorter distances traveled in WT animals, we provide strong evidence that running less does not translate into the absence of running-induced neurogenic response. The data confirm our hypothesis that ACE2 is required for the exercise-dependent effect.
To define a mechanism around ACE2, we examined the angiotensin peptides and targeted Ang II by blocking AT1, and Ang-(1–7) by deleting Mas. Manipulation of angiotensin peptide levels during running might impact cell proliferation in the DG. Recent studies have shown that physical exercise stimulates levels of circulating Ang II in rats [40, 41]. A pro-mitotic effect was also observed following intravascular Ang II infusion into rats, which was reversed by AT1 blockade with losartan [41]. Our data show that AT1 activation is required for running-induced proliferation. TEL treatment to some extent blocked the pro-mitotic effect of running in WT mice confirming a stimulating role of AT1. However, TEL treatment did not rescue running-induced proliferation in ACE2-deficient mice arguing for AT1-independent mechanisms in this mouse model. Circulating Ang-(1–7) levels are elevated following physical activity [42]. Using Mas-deficient mice, our data reveal the typical neurogenic response to running and thus argue that a lowered synthesis rate of Ang-(1–7), as observed in ACE2-deficient mice [14], is not responsible for the lack of running effect. Furthermore, we did not observe changes in the phenotype distribution of newly generated cells in this model compared with WT, as was previously reported based on increased numbers of DCX+ cells in the DG of Mas-deficient mice [17]. Besides technical differences in the analysis of neurogenesis (BrdU vs. DCX), the known variation in the neurogenic response between mouse strains (mixed 129/C57BL/6 background in Freund et al. vs. C57BL/6 in our study [43]) may affect the outcome. Overall, add-on stimuli, such as TEL or Trp diet, did not lead to increased numbers of progenitor cells for ACE2-deficient mice after running at any time. An alternative pathway could be Ang II-mediated stimulation of AT2, which has been shown to contribute to increased neurogenesis induced by heat acclimatization in both the hippocampus and subventricular zone of rats [44]. Furthermore, other peptide substrates of ACE2, such as apelin, dynorphin A, or des-Arg9-bradykinin [45] may mediate the effects on running-induced cell proliferation. Future experiments may clarify the suggested molecular mechanisms involved in ACE2-mediated neurogenesis.
We discovered that ACE2 plays an important role in 5-HT metabolism. Using Tph2−/− mice, we have previously established that 5-HT is required for the pro-mitotic effect of running [5]. Here, we discovered similar results in that permanent depletion of ACE2 did not change baseline neurogenesis, but abolished the effect of running on cell proliferation in the adult hippocampus. However, experimentally enhanced Trp/5-HT levels in the brain did not affect proliferation at baseline nor rescue the neurogenic response to running in ACE2-deficient mice, arguing for a 5-HT-independent phenotype. The beneficial aspect of running [3, 4] was seen in all WT groups. However, increased cell proliferation was attenuated following Trp diet. The mechanism behind this effect of Trp remains elusive, but may involve a dysbalance of AAs in the brain after Trp diet. Yet, cell proliferation at baseline was not affected when fed Trp diet. Alterations in running performance under conditions of changes in 5-HT levels might be due to lack of motivation or early satiation. Both are unlikely, since the running performance pattern showed constant running at night.
Our data highlight a novel pathway, in that ACE2 affects the proliferative phase in adult hippocampal neurogenesis following physical exercise. Exercise has been established as a positive neuromodulator, and recent studies focus on the neuroprotective effects [46]. Furthermore, clinical research document mood improvements in patients treated with drugs that target the Ang II/ACE/AT1 axis, e.g., ACE inhibitors [47], which are primarily used for the treatment of hypertension. Further studies will elucidate whether stimulation of ACE2 activity [48] or administration of the recombinant enzyme [49] might positively affect cell proliferation and adult neurogenesis and in turn detain the progression of depressive disorders.
References
Altman J, Das GD (1965) Autoradiographic and histological evidence of postnatal hippocampal neurogenesis in rats. J Comp Neurol 124(3):319–335
Kempermann G, Kuhn HG, Gage FH (1997) More hippocampal neurons in adult mice living in an enriched environment. Nature 386(6624):493–495
van Praag H, Kempermann G, Gage FH (1999) Running increases cell proliferation and neurogenesis in the adult mouse dentate gyrus. Nat Neurosci 2(3):266–270
Kronenberg G, Reuter K, Steiner B et al (2003) Subpopulations of proliferating cells of the adult hippocampus respond differently to physiologic neurogenic stimuli. J Comp Neurol 467(4):455–463
Klempin F, Beis D, Mosienko V et al (2013) Serotonin is required for exercise-induced adult hippocampal neurogenesis. J Neurosci 33(19):8270–8275
Chieffi S, Messina G, Villano I et al (2017) Exercise influence on hippocampal function: possible involvement of orexin-A. Front Physiol 8:85
Fabel K, Fabel K, Tam B et al (2003) VEGF is necessary for exercise-induced adult hippocampal neurogenesis. Eur J Neurosci 18(10):2803–2812
Trejo JL, Carro E, Torres-Aleman I (2001) Circulating insulin-like growth factor I mediates exercise-induced increases in the number of new neurons in the adult hippocampus. J Neurosci 21(5):1628–1634
Turner AJ, Hooper NM (2002) The angiotensin-converting enzyme gene family: genomics and pharmacology. Trends Pharmacol Sci 23(4):177–183
Santos RA, Simoes e Silva AC, Maric C et al (2003) Angiotensin-(1–7) is an endogenous ligand for the G protein-coupled receptor Mas. Proc Natl Acad Sci USA 100(14):8258–8263
Rabelo LA, Alenina N, Bader M (2011) ACE2-angiotensin-(1–7)-Mas axis and oxidative stress in cardiovascular disease. Hypertens Res 34(2):154–160
Santos RA, Ferreira AJ, Verano-Braga T et al (2013) Angiotensin-converting enzyme 2, angiotensin-(1–7) and Mas: new players of the renin–angiotensin system. J Endocrinol 216(2):R1–R17
Gironacci MM, Cerniello FM, Longo Carbajosa NA et al (2014) Protective axis of the renin–angiotensin system in the brain. Clin Sci (Lond) 127(5):295–306
Xu P, Sriramula S, Lazartigues E (2011) ACE2/ANG-(1–7)/Mas pathway in the brain: the axis of good. Am J Physiol Regul Integr Comp Physiol 300(4):R804–R817
Bunnemann B, Fuxe K, Metzger R et al (1990) Autoradiographic localization of mas proto-oncogene mRNA in adult rat brain using in situ hybridization. Neurosci Lett 114(2):147–153
Doobay MF, Talman LS, Obr TD et al (2007) Differential expression of neuronal ACE2 in transgenic mice with overexpression of the brain renin–angiotensin system. Am J Physiol Regul Integr Comp Physiol 292(1):R373–R381
Freund M, Walther T, von und Halbach BO (2012) Immunohistochemical localization of the angiotensin-(1–7) receptor Mas in the murine forebrain. Cell Tissue Res 348(1):29–35
Kuba K, Imai Y, Rao S et al (2005) A crucial role of angiotensin converting enzyme 2 (ACE2) in SARS coronavirus-induced lung injury. Nat Med 11(8):875–879
Camargo SM, Singer D, Makrides V et al (2009) Tissue-specific amino acid transporter partners ACE2 and collectrin differentially interact with Hartnup mutations. Gastroenterology 136(3):872–882
Kowalczuk S, Broer A, Tietze N et al (2008) A protein complex in the brush-border membrane explains a Hartnup disorder allele. FASEB J 22(8):2880–2887
Hashimoto T, Perlot T, Rehman A et al (2012) ACE2 links amino acid malnutrition to microbial ecology and intestinal inflammation. Nature 487(7408):477–481
Singer D, Camargo SM, Ramadan T et al (2012) Defective intestinal amino acid absorption in Ace2 null mice. Am J Physiol Gastrointest Liver Physiol 303(6):G686–G695
Duelli R, Enerson BE, Gerhart DZ et al (2000) Expression of large amino acid transporter LAT1 in rat brain endothelium. J Cereb Blood Flow Metab 20(11):1557–1562
Alenina N, Klempin F (2015) The role of serotonin in adult hippocampal neurogenesis. Behav Brain Res 277:49–57
Crackower MA, Sarao R, Oudit GY et al (2002) Angiotensin-converting enzyme 2 is an essential regulator of heart function. Nature 417(6891):822–828
Walther T, Balschun D, Voigt JP et al (1998) Sustained long term potentiation and anxiety in mice lacking the Mas protooncogene. J Biol Chem 273(19):11867–11873
Marlatt MW, Lucassen PJ, van Praag H (2010) Comparison of neurogenic effects of fluoxetine, duloxetine and running in mice. Brain Res 1341:93–99
Yamaguchi T, Sawada M, Kato T et al (1981) Demonstration of tryptophan 5-monooxygenase activity in human brain by high sensitive high-performance liquid chromatography with fluorometric detection. Biochem Int 2:295–303
Mosienko V, Matthes S, Hirth N et al (2014) Adaptive changes in serotonin metabolism preserve normal behavior in mice with reduced TPH2 activity. Neuropharmacology 85:73–80
Kempermann G (2004) Milestones of neuronal development in the adult hippocampus. Trends Neurosci 27(8):447–452
Gohlke P, Weiss S, Jansen A et al (2001) AT1 receptor antagonist telmisartan administered peripherally inhibits central responses to angiotensin II in conscious rats. J Pharmacol Exp Ther 298(1):62–70
Alenina N, Kikic D, Todiras M et al (2009) Growth retardation and altered autonomic control in mice lacking brain serotonin. Proc Natl Acad Sci USA 106(25):10332–10337
Holmes MM, Galea LA, Mistlberger RE et al (2004) Adult hippocampal neurogenesis and voluntary running activity: circadian and dose-dependent effects. J Neurosci Res 76(2):216–222
Motta-Santos D, Dos Santos RA, Oliveira M et al (2016) Effects of ACE2 deficiency on physical performance and physiological adaptations of cardiac and skeletal muscle to exercise. Hypertens Res 39(7):506–512
Moon HY, Becke A, Berron D et al (2016) Running-induced systemic cathepsin B secretion is associated with memory function. Cell Metab 24(2):332–340
Bindom SM, Lazartigues E (2009) The sweeter side of ACE2: physiological evidence for a role in diabetes. Mol Cell Endocrinol 302(2):193–202
Dias-Peixoto MF, Santos RA, Gomes ER et al (2008) Molecular mechanisms involved in the angiotensin-(1–7)/Mas signaling pathway in cardiomyocytes. Hypertension (Dallas, Tex: 1979) 52(3):542–548
Morales MG, Abrigo J, Acuna MJ et al (2016) Angiotensin-(1–7) attenuates disuse skeletal muscle atrophy in mice via its receptor, Mas. Dis Models Mech 9(4):441–449
Santos SH, Fernandes LR, Mario EG et al (2008) Mas deficiency in FVB/N mice produces marked changes in lipid and glycemic metabolism. Diabetes 57(2):340–347
Leite LH, Santiago HP, de Almeida RS et al (2013) Implications of angiotensin II in central nervous system on exercise performance. Curr Protein Pept Sci 14(8):711–720
Mukuda T, Koyama Y, Hamasaki S et al (2014) Systemic angiotensin II and exercise-induced neurogenesis in adult rat hippocampus. Brain Res 1588:92–103
Guimaraes GG, Santos SH, Oliveira ML et al (2012) Exercise induces renin–angiotensin system unbalance and high collagen expression in the heart of Mas-deficient mice. Peptides 38(1):54–61
Merritt JR, Rhodes JS (2015) Mouse genetic differences in voluntary wheel running, adult hippocampal neurogenesis and learning on the multi-strain-adapted plus water maze. Behav Brain Res 280:62–71
Umschweif G, Shabashov D, Alexandrovich AG et al (2014) Neuroprotection after traumatic brain injury in heat-acclimated mice involves induced neurogenesis and activation of angiotensin receptor type 2 signaling. J Cereb Blood Flow Metab 34(8):1381–1390
Vickers C, Hales P, Kaushik V et al (2002) Hydrolysis of biological peptides by human angiotensin-converting enzyme-related carboxypeptidase. J Biol Chem 277(17):14838–14843
Chieffi S, Messina G, Villano I et al (2017) Neuroprotective effects of physical activity: evidence from human and animal studies. Front Neurol 8:188
Wright JW, Harding JW (2011) Brain renin–angiotensin—a new look at an old system. Prog Neurobiol 95(1):49–67
Hernandez AF, Harrington RA (2008) Comparative effectiveness of angiotensin-converting-enzyme inhibitors: is an ACE always an ace? CMAJ 178(10):1316–1319
Imai Y, Kuba K, Rao S et al (2005) Angiotensin-converting enzyme 2 protects from severe acute lung failure. Nature 436(7047):112–116
Acknowledgements
This work was supported by Deutsche Forschungsgemeinschaft (DFG) award KL 2805/1-1 to F.K., the Brazilian fellowship BJT 407352/2013-9 to N.A., the German Academic Exchange Service (DAAD)/the Brazilian National Council for Scientific and Technological Development (CNPq) program PROBRAL to N.A. and R.A.S.S, and RSF Grant 14-50-00069 to N.A. The authors thank Susanne da Costa Goncalves, Sabine Grüger, Charlene Memler, and Thorsten Riepenhausen for their excellent technical assistance.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare no competing financial interests.
Rights and permissions
About this article
Cite this article
Klempin, F., Mosienko, V., Matthes, S. et al. Depletion of angiotensin-converting enzyme 2 reduces brain serotonin and impairs the running-induced neurogenic response. Cell. Mol. Life Sci. 75, 3625–3634 (2018). https://doi.org/10.1007/s00018-018-2815-y
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00018-018-2815-y