Abstract
Objective
Preintervention thrombus burden in the infarct-related artery is an independent predictor of no-reflow and adverse outcomes in coronary artery disease. The role of D-dimers in the acute phase of ST-elevated myocardial infarction (STEMI) during primary percutaneous coronary intervention (PCI) has not been fully elucidated. We aimed to investigate the predictive value of serum D-dimer levels on the outcome of patients with STEMI.
Methods and results
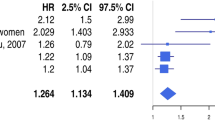
A total of 266 consecutive patients presenting with STEMI within the first 12 h of symptom onset were included in this study. Patients were divided into two groups based on the postinterventional Thrombolysis In Myocardial Infarction (TIMI) flow grade score. Postinterventional TIMI grades of 0, 1, or 2 were defined as no-reflow (group 1) and angiographic success was defined as TIMI 3 flow (group 2). D-dimer levels were significantly higher in patients with postinterventional no-reflow than in patients with postinterventional TIMI grade 3 flow (686 ± 236 μg/ml–418 ± 164 μg/ml, p < 0.001). Multivariate logistic regression analysis showed that D-dimer level was an independent predictor of postinterventional no-reflow (OR: 1.005; 95 % CI: 1.003–1.007; p < 0.001) and in-hospital major adverse cardiovascular events (MACE; OR: 1.002; 95 % CI: 1.000–1.004; p = 0.029). Receiver operator characteristics analysis provided a cut-off value of 549 μg/ml for D-dimer for predicting no-reflow with an 83 % sensitivity and an 81 % specificity, and 544 μg/ml for predicting in-hospital MACE with a 69 % sensitivity and a 67 % specificity.
Conclusion
In conclusion, D-dimer levels measured on admission may be an independent predictor of no-reflow, which is also a predictor of adverse outcomes in patients with STEMI.
Zusammenfassung
Ziel
Die prävinterventionelle Thrombuslast in der vom Infarkt betroffenen Arterie stellt einen unabhängigen Prädiktor des No-Reflow-Phänomens und ungünstiger Ereignisse bei koronarer Herzkrankheit dar. Die Rolle des D-Dimers in der akuten Phase des ST-Strecken-Hebungs-Infarkts (STEMI) bei primärer perkutaner Koronarintervention (PCI) ist noch nicht vollständig geklärt. Ziel der Studie war es, den prädiktiven Wert der D-Dimer-Serumspiegel für den Verlauf bei Patienten mit STEMI zu untersuchen.
Methoden und Ergebnisse
Insgesamt wurden 266 Patienten mit STEMI, die sich innerhalb der ersten 12 h nach Beginn der Symptome vorstellten, konsekutiv in die Studie aufgenommen. Die Patienten wurden gemäß ihrem postinterventionellen Grad des Blutflusses nach TIMI („thrombolysis in myocardial infarction“) in 2 Gruppen aufgeteilt. Ein postinterventioneller TIMI-Grad von 0, 1 oder 2 wurde als No-Reflow-Phänomen (Gruppe 1) und ein angiographischer Erfolg als TIMI-Fluss von 3 definiert (Gruppe 2). Bei Patienten mit postinterventionellem No-Reflow-Phänomen waren die D-Dimer-Werte signifikant höher als bei Patienten mit postinterventionellem Fluss von TIMI-Grad 3 (686 ± 236 μg/ml–418 ± 164 μg/ml; p < 0,001). Die multivariate logistische Regressionsanalyse ergab, dass der D-Dimer-Spiegel ein unabhängiger Prädiktor für ein postinterventionelles No-Reflow-Phänomen (OR: 1,005; 95%-KI: 1,003–1,007; p < 0,001) und für größere unerwünschte kardiovaskuläre Ereignisse („major adverse cardiovascular events“, MACE) während des stationären Aufenthalts (OR: 1,002; 95%-KI: 1,000–1,004; p = 0,029) war. Nach der Receiver-operating-characteristics-Analyse ergab sich ein D-Dimer-Grenzwert von 549 μg/ml, um ein No-Reflow-Phänomen mit einer Sensitivität von 83 % und einer Spezifität von 81 % vorherzusagen, und ein D-Dimer-Grenzwert von 544 μg/ml, um MACE während des stationären Aufenthalts mit einer Sensitivität von 69 % und einer Spezifität von 67 % vorherzusagen.
Schlussfolgerung
Fazit ist, dass der bei Aufnahme bestimmte D-Dimer-Spiegel möglicherweise ein unabhängiger Prädiktor für ein No-Reflow-Phänomen ist, welches auch einen Prädiktor für unerwünschte Ereignisse bei Patienten mit STEMI darstellt.



Similar content being viewed by others
References
Kaya MG, Uyarel H, Akpek M et al (2012) Prognostic value of uric acid in patients with ST-elevated myocardial infarction undergoing primary coronary intervention. Am J Cardiol 109:486–491
Cura F, Albertal M, Thierer J et al (2011) Quality of myocardial reperfusion according to ischemic time and infarcted territory. Coron Artery Dis 22:92–95
Buyukkaya E, Poyraz F, Karakas MF et al (2013) Usefulness of monocyte chemoattractant protein-1 to predict no-reflow and three-year mortality in patients with ST-Segment Elevation myocardial infarction undergoing primary percutaneous coronary intervention. Am J Cardiol 112:187–193
Akpek M, Kaya MG, Lam YY et al (2012) Relation of neutrophil/lymphocyte ratio to coronary flow to in-hospital major adverse cardiac events in patients with ST-elevated myocardial infarction undergoing primary coronary intervention. Am J Cardiol 110:621–627
Tripodi A (2011) D-dimer testing in laboratory practice. Clin Chem 57:1256–1262
Yip HK, Chen MC, Chang HW et al (2002) Angiographic morphologic features of infarct-related arteries and timely reperfusion in acute myocardial infarction: predictors of slow-flow and noreflow phenomenon. Chest 122:1322–1332
Sianos G, Papafaklis MI, Daemen J et al (2007) Angiographic stent thrombosis after routine use of drug-eluting stents in ST-segment elevation myocardial infarction: the importance of thrombus burden. J Am Coll Cardiol 50:573–583
Kirma C, Izgi A, Dundar C et al (2008) Clinical and procedural predictors of no-reflow phenomenon after primary percutaneous coronary interventions: experience at a single center. Circ J 72:716–721
Hochuli M, Duewell S, Frauchiger B (2007) Quantitative d-dimer levels and the extent of venous thromboembolism in CT angiography and lower limb ultrasonography. Vasa 36:267–274
Rezkalla SH, Dharmashankar KC, Abdalrahman IB et al (2010) No-reflow phenomenon following percutaneous coronary intervention for acute myocardial infarction: incidence, outcome, and effect of pharmacologic therapy. J Interv Cardiol 23:429–436
Niccoli G, Lanza GA, Spaziani C et al (2007) Baseline systemic inflammatory status and no-reflow phenomenon after percutaneous coronary angioplasty for acute myocardial infarction. Int J Cardiol 117:306–311
Sarli B, Baktir AO, Saglam H et al (2013) Mean platelet volume is associated with poor postinterventional myocardial blush grade in patients with ST-segment elevation myocardial infarction. Coron Artery Dis 24:285–289
Kırma C, Oduncu V, Tanalp AC et al (2011) Primary angioplasty in a high-volume tertiary center in Turkey: in-hospital clinical outcomes of 1625 patients. Turk Kardiyol Dern Ars 39:300–307
Niccoli G, Burzotta F, Galiuto L et al (2009) Myocardial no-reflow in humans. J Am Coll Cardiol 54:281–292
Resnic FS, Wainstein M, Lee MK et al (2003) No-reflow is an independent predictor of death and myocardial infarction after percutaneous coronary intervention. Am Heart J 145:42–46
Topol EJ, Yadav JS (2000) Recognition of the importance of embolization in atherosclerotic vascular disease. Circulation 101:570–580
Ramjane K, Han L, Jin C (2008) The diagnosis and treatment of the no-reflow phenomenon in patients with myocardial infarction undergoing percutaneous coronary intervention. Exp Clin Cardiol 13:121–128
Nallamothu BK, Bradley EH, Krumholz HM (2007) Time to treatment in primary percutaneous coronary intervention. N Engl J Med 357:1631–1638
Niccoli G, Giubilato S, Russo E et al (2008) Plasma levels of thromboxane A2 on admission are associated with no-reflow after primary percutaneous coronary intervention. Eur Heart J 29:1843–1850
Niccoli G, Lanza GA, Shaw S (2006) Endothelin-1 and acute myocardial infarction: a no-reflow mediator after successful percutaneous myocardial revascularization. Eur Heart J 27:1793–1798
Brosh D, Assali AR, Mager A et al (2007) Effect of no-reflow during primary percutaneous coronary intervention for acute myocardial infarction on six-month mortality. Am J Cardiol 99:442–445
Henriques JP, Zijlstra F, Hof AW van ‘t et al (2003) Angiographic assessment of reperfusion in acute myocardial infarction by myocardial blush grade. Circulation 107:2115–2119
Gibson CM, Cannon CP, Murphy SA et al (2002) Relationship of the TIMI myocardial perfusion grades, flow grades, frame count, and percutaneous coronary intervention to long-term outcomes after thrombolytic administration in acute myocardial infarction. Circulation 105:1909–1913
Limbruno U, De Carlo M, Pistolesi S et al (2005) Distal embolization during primary angioplasty: histopathologic features and predictability. Am Heart J 150:102–108
Skyschally A, Leineweber K, Gres P et al (2006) Coronary microembolization. Basic Res Cardiol 101:373–382
Hori M, Inoue M, Kitakaze M et al (1986) Role of adenosine in hyperemic response of coronary blood flow in microembolization. Am J Physiol 250:H509–H518
Goldhaber SZ, Bounameaux H (2012) Pulmonary embolism and deep vein thrombosis. Lancet 379:1835–1846
Turker Y, Dogan A, Ozaydin M et al (2010) Association of thrombotic and fibrinolytic factors with severity of culprit lesion in patients with acute coronary syndromes without ST elevation. South Med J 103:289–294
Saigo M, Hsue PY, Waters DD (2004) Role of thrombotic and fibrinolytic factors in acute coronary syndromes. Prog Cardiovasc Dis 46:524–538
Akgul O, Uyarel H, Pusuroglu H et al (2013) Predictive value of elevated D-dimer in patients undergoing primary angioplasty for ST elevation myocardial infarction. Blood Coagul Fibrinolysis 24:704–710
Davies MJ (1990) A macro and micro view of coronary vascular insult in ischemic heart disease. Circulation 82:II38–II46
Compliance with ethical guidelines
Conflict of interest. B. Sarli, M. Akpek, A.O. Baktir, O. Sahin, H. Saglam, H. Arinc, H, Odabasi, S. Dogan, S. Kurtul, Y. Dogan, and M.G. Kaya state that there are no conflicts of interest. All studies on humans described in the present manuscript were carried out with the approval of the responsible ethics committee and in accordance with national law and the Helsinki Declaration of 1975 (in its current, revised form). Informed consent was obtained from all patients included in studies.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Sarli, B., Akpek, M., Baktir, A. et al. Impact of D-dimer level on postinterventional coronary flow and in-hospital MACE in ST-segment elevation myocardial infarction. Herz 40, 507–513 (2015). https://doi.org/10.1007/s00059-013-4029-2
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00059-013-4029-2
Keywords
- D-dimer
- ST-segment elevation myocardial infarction
- Primary percutaneous coronary intervention
- TIMI flow grade
- Major adverse cardiovascular events