Abstract
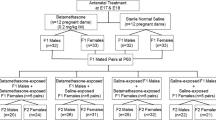
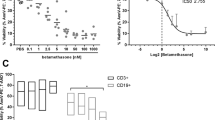
Prenatal steroids have an undisputed positive effect of decreasing neonatal morbidity and mortality by improving fetal lung maturation. Some concerns have been raised on long-term consequences on the hypothalamic–pituitary–adrenal axis and cognition, but there are no studies addressing effects on the immune system. The thymus is an essential organ for the development and selection of T cells, and thymocytes are extremely sensitive to steroids. Using a mouse model for prenatal steroid administration, we show here that betamethasone treatment to the mother has a profound effect on the thymus of the offspring. We find the thymus volume reduced, affecting mostly the developing CD4+ CD8+ double-positive thymocytes and a compensatory accelerated transition of the earlier stages to replenish the depleted compartment. This effect lasts for at least 3 days, which correspond to a very relevant period for the selection of the T cell repertoire. Moreover, we show that low doses of betamethasone have similar effects on human thymocytes in vitro. Therefore, further studies are needed to analyze possible long-term consequences of this treatment on the immune system of the offspring.
Key message
-
Betamethasone administered to the mother before birth reaches the fetal thymus.
-
Prenatal betamethasone results in massive loss of developing thymocytes.
-
The effects of betamethasone on thymus development are visible for several days.
-
Human thymocytes are also sensitive to low doses of betamethasone.
-
Altered thymocyte development around birth may have an effect on the immune system.







Similar content being viewed by others
References
Miracle X, Di Renzo GC, Stark A, Fanaroff A, Carbonell-Estrany X, Saling E, Coordinators Of World Association of Perinatal Medicine Prematurity Working G (2008) Guideline for the use of antenatal corticosteroids for fetal maturation. J Perinatal Med 36:191–196
Alexander N, Rosenlocher F, Stalder T, Linke J, Distler W, Morgner J, Kirschbaum C (2012) Impact of antenatal synthetic glucocorticoid exposure on endocrine stress reactivity in term-born children. J Clin Endoctinol Metab 97:3538–3544
Huang WL, Beazley LD, Quinlivan JA, Evans SF, Newnham JP, Dunlop SA (1999) Effect of corticosteroids on brain growth in fetal sheep. Obstet Gynecol 94:213–218
Uno H, Lohmiller L, Thieme C, Kemnitz JW, Engle MJ, Roecker EB, Farrell PM (1990) Brain damage induced by prenatal exposure to dexamethasone in fetal rhesus macaques. I. Hippocampus. Brain Res Dev Brain Res 53:157–167
Cohen JJ, Duke RC (1984) Glucocorticoid activation of a calcium-dependent endonuclease in thymocyte nuclei leads to cell death. J Immunol 132:38–42
Ashwell JD, Lu FW, Vacchio MS (2000) Glucocorticoids in T cell development and function. Ann Rev Immunol 18:309–345
Mittelstadt PR, Monteiro JP, Ashwell JD (2012) Thymocyte responsiveness to endogenous glucocorticoids is required for immunological fitness. J Clin Invest 122:2384–2394
van den Brandt J, Luhder F, McPherson KG, de Graaf KL, Tischner D, Wiehr S, Herrmann T, Weissert R, Gold R, Reichardt HM (2007) Enhanced glucocorticoid receptor signaling in T cells impacts thymocyte apoptosis and adaptive immune responses. Am J Pathol 170:1041–1053
Pole JD, Mustard CA, To T, Beyene J, Allen AC (2009) Antenatal steroid therapy for fetal lung maturation: is there an association with childhood asthma? J Asthma 46:47–52
Christensen HD, Sienko AE, Rayburn WF, Gonzalez CL, Coleman FH (1997) A placebo-controlled, blinded comparison between betamethasone and dexamethasone to enhance lung maturation in the fetal mouse. J Soc Gynecol Investig 4:130–134
Stewart JD, Sienko AE, Gonzalez CL, Christensen HD, Rayburn WF (1998) Placebo-controlled comparison between a single dose and a multidose of betamethasone in accelerating lung maturation of mice offspring. Am J Obstet Gynecol 179:1241–1247
Rodewald HR, Fehling HJ (1998) Molecular and cellular events in early thymocyte development. Adv Immunol 69:1–112
McKinlay CJ, Crowther CA, Middleton P, Harding JE (2012) Repeat antenatal glucocorticoids for women at risk of preterm birth: a Cochrane Systematic Review. Am J Obstet Gynecol 206:187–194
Roberts D, Dalziel S (2006) Antenatal corticosteroids for accelerating fetal lung maturation for women at risk of preterm birth. Cochrane Database System Rev: CD004454
Benediktsson R, Lindsay RS, Noble J, Seckl JR, Edwards CR (1993) Glucocorticoid exposure in utero: new model for adult hypertension. Lancet 341:339–341
Sugden MC, Langdown ML, Munns MJ, Holness MJ (2001) Maternal glucocorticoid treatment modulates placental leptin and leptin receptor expression and materno-fetal leptin physiology during late pregnancy, and elicits hypertension associated with hyperleptinaemia in the early-growth-retarded adult offspring. Eur J Endocrinol 145:529–539
Antonow-Schlorke I, Schwab M, Li C, Nathanielsz PW (2003) Glucocorticoid exposure at the dose used clinically alters cytoskeletal proteins and presynaptic terminals in the fetal baboon brain. J Physiol 547:117–123
Huang WL, Harper CG, Evans SF, Newnham JP, Dunlop SA (2001) Repeated prenatal corticosteroid administration delays astrocyte and capillary tight junction maturation in fetal sheep. Int J Dev Neurosci: Off J Int Soc Dev Neurosci 19:487–493
Lindsay RS, Lindsay RM, Waddell BJ, Seckl JR (1996) Prenatal glucocorticoid exposure leads to offspring hyperglycaemia in the rat: studies with the 11 beta-hydroxysteroid dehydrogenase inhibitor carbenoxolone. Diabetologia 39:1299–1305
Nyirenda MJ, Lindsay RS, Kenyon CJ, Burchell A, Seckl JR (1998) Glucocorticoid exposure in late gestation permanently programs rat hepatic phosphoenolpyruvate carboxykinase and glucocorticoid receptor expression and causes glucose intolerance in adult offspring. J Clin Invest 101:2174–2181
Kavelaars A, van der Pompe G, Bakker JM, van Hasselt PM, Cats B, Visser GH, Heijnen CJ (1999) Altered immune function in human newborns after prenatal administration of betamethasone: enhanced natural killer cell activity and decreased T cell proliferation in cord blood. Pediatr Res 45:306–312
Michie CA, Hasson N, Tulloh R (1998) The neonatal thymus and antenatal steroids. Archives of disease in childhood. Fetal Neonatal Ed 79:F159
Vacchio MS, Papadopoulos V, Ashwell JD (1994) Steroid production in the thymus: implications for thymocyte selection. J Exp Med 179:1835–1846
Lu FW, Yasutomo K, Goodman GB, McHeyzer-Williams LJ, McHeyzer-Williams MG, Germain RN, Ashwell JD (2000) Thymocyte resistance to glucocorticoids leads to antigen-specific unresponsiveness due to “holes” in the T cell repertoire. Immunity 12:183–192
Tolosa E, King LB, Ashwell JD (1998) Thymocyte glucocorticoid resistance alters positive selection and inhibits autoimmunity and lymphoproliferative disease in MRL-lpr/lpr mice. Immunity 8:67–76
Tolosa E, Ashwell JD (1999) Thymus-derived glucocorticoids and the regulation of antigen-specific T-cell development. Neuroimmunomodulation 6:90–96
Fletcher AL, Lowen TE, Sakkal S, Reiseger JJ, Hammett MV, Seach N, Scott HS, Boyd RL, Chidgey AP (2009) Ablation and regeneration of tolerance-inducing medullary thymic epithelial cells after cyclosporine, cyclophosphamide, and dexamethasone treatment. J Immunol 183:823–831
Jung C, Ho JT, Torpy DJ, Rogers A, Doogue M, Lewis JG, Czajko RJ, Inder WJ (2011) A longitudinal study of plasma and urinary cortisol in pregnancy and postpartum. J Clin Endocrinol Metab 96:1533–1540
Cottrell EC, Holmes MC, Livingstone DE, Kenyon CJ, Seckl JR (2012) Reconciling the nutritional and glucocorticoid hypotheses of fetal programming. FASEB J 26:1866–1874
Khulan B, Drake AJ (2012) Glucocorticoids as mediators of developmental programming effects. Best Practice Res Clin Endocrinol Metab 26:689–700
Solano ME, Jago C, Pincus MK, Arck PC (2011) Highway to health; or how prenatal factors determine disease risks in the later life of the offspring. J Reprod Immunol 90:3–8
Gluckman PD, Hanson MA, Cooper C, Thornburg KL (2008) Effect of in utero and early-life conditions on adult health and disease. N Engl J Med 359:61–73
Pincus-Knackstedt MK, Joachim RA, Blois SM, Douglas AJ, Orsal AS, Klapp BF, Wahn U, Hamelmann E, Arck PC (2006) Prenatal stress enhances susceptibility of murine adult offspring toward airway inflammation. J Immunol 177:8484–8492
Seckl JR (2004) Prenatal glucocorticoids and long-term programming. Eur J Endocrinol 151(Suppl 3):U49–U62
Crudo A, Suderman M, Moisiadis VG, Petropoulos S, Kostaki A, Hallett M, Szyf M, Matthews SG (2013) Glucocorticoid programming of the fetal male hippocampal epigenome. Endocrinology 154:1168–1180
Seckl JR, Holmes MC (2007) Mechanisms of disease: glucocorticoids, their placental metabolism and fetal ‘programming’ of adult pathophysiology. Nature Clin Pract Endocrinol Metab 3:479–488
Acknowledgments
We would like to thank the Flow Cytometry Core Facility at the UKE, Tobias Mummert, Thomas Andreas, and Corinna Kulicke for excellent technical help. This project is supported by the Excellence Initiative of the Hamburg Research Foundation (LEXI).
Conflict of interest
The authors reported no potential conflicts of interest.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Diepenbruck, I., Much, C.C., Krumbholz, A. et al. Effect of prenatal steroid treatment on the developing immune system. J Mol Med 91, 1293–1302 (2013). https://doi.org/10.1007/s00109-013-1069-2
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00109-013-1069-2