Abstract
Background
Oxygen saturation monitoring for children receiving respiratory support is standard worldwide. No randomised clinical trials have compared peripheral oxygen saturation (SpO2) targets for critically ill children. The harm of interventions to raise SpO2 to > 94% may exceed their benefits.
Methods
We undertook an open, parallel-group randomised trial of children > 38 weeks completed gestation and < 16 years of age receiving invasive or non-invasive respiratory support and supplemental oxygen who were admitted urgently to one of three paediatric intensive care units. A ‘research without prior consent’ approach was employed. Children were randomly assigned to a liberal oxygenation group (SpO2 targets > 94%) or a conservative oxygenation group (SpO2 = 88–92% inclusive). Outcomes were measures of feasibility: recruitment rate, protocol adherence and acceptability, between-group separation of SpO2 and safety. The Oxy-PICU trial was registered before recruitment: ClinicalTrials.gov identifier NCT03040570.
Results
A total of 159 children met the inclusion criteria, of whom 119 (75%) were randomised between April and July 2017, representing a rate of 10 patients per month per site. The mean time to randomisation from first contact with an intensive care team was 1.9 (SD 2.2) h. Consent to continue in the study was obtained in 107 cases (90%); the children’s parents/legal representatives were supportive of the consent process. The median (interquartile range, IQR) of time-weighted individual mean SpO2 was 94.9% (92.6–97.1) in the conservative oxygenation group and 97.5% (96.2–98.4) in the liberal group [difference 2.7%, 95% confidence interval (95% CI) 1.3–4.0%, p < 0.001]. Median (IQR) time-weighted individual mean FiO2 was 0.28 (0.24–0.37) in the conservative group and 0.37 (0.30–0.42) in the liberal group (difference 0.08, 95% CI 0.03–0.13, p < 0.001). There were no significant between-group differences in length of stay, duration of organ support or mortality. Two prespecified serious adverse events (cardiac arrests) occurred, both in the liberal oxygenation group.
Conclusion
A definitive clinical trial of peripheral oxygen saturation targets is feasible in critically ill children.
Similar content being viewed by others
Introduction
A main aim of resuscitation and intensive care is to maintain appropriate and safe levels of tissue oxygenation [1,2,3]. However, as it is difficult to directly measure tissue oxygen, estimates of peripheral oxygen saturation (SpO2) and arterial partial pressure are typically substituted. Invasive mechanical ventilation with supplemental oxygen to maintain oxygen saturation and partial pressure is the most common organ support provided in paediatric intensive care units (PICU). Around 70% of UK PICU admissions are invasively mechanically ventilated, amounting to 14–15,000 children annually [4]. Globally, the figure is unknown, but is in the hundreds of thousands. Despite these numbers, there is no high-quality evidence from randomised clinical trials (RCTs) to inform the optimal level of oxygen saturation for critically ill children receiving mechanical ventilation.
Current practice relating to oxygen saturation targets varies widely in paediatric and adult intensive care units [5, 6]. Clinicians prevent severe hypoxia wherever possible, but beyond this there is little consensus [7]. Indeed, a fear of hypoxia leads many to target supra-normal values. We previously described supra-physiological levels of oxygenation as the norm on PICU; around one-third of all recorded SpO2 values were 100% and more than 60% of values were over 95% [8].
Associations between high levels of arterial oxygenation and worse outcomes have been described post-cardiac arrest [9, 10], during extra-corporeal oxygenation for congenital heart disease [11], following stroke [12], and in respiratory failure [13]. When added to the known risks of severe hypoxia, a ‘U-shaped’ relationship between arterial oxygenation and risk of death emerges [3]. We observed an excess mortality (both crude and adjusted) at extremes of oxygenation in 7410 critically ill children [14]. A complex U-shaped relationship between oxygenation and outcome is biologically feasible and may arise from the balance between harm from hypoxic injury at one extreme and a combination of increased oxygen free radical damage and iatrogenic injury from more aggressive treatments at the other [15].
Clinical trials of oxygen targets in extremely premature infants have shown that they influence survival rates, retinopathy rates, and costs, with large RCTs comparing lower (85–89%) with higher SpO2 targets (91–95%) [16, 17]. Unexpectedly, an increased risk of death [relative risk 1.45; 95% confidence interval (CI), 1.15–1.84; p = 0.002] was observed with the lower SpO2 targets [17]. In contrast, in adult critical illness, small trials indicate a possible benefit of lower oxygenation targets [18, 19]. No benefit or significant harm with supplemental oxygen has been demonstrated in adults with ST elevation myocardial infarction [20,21,22].
The only paediatric trial data—in non-critically ill children with bronchiolitis—demonstrate equivalent safety of a SpO2 target of > 90% when compared to > 94%. Later hospital discharges were seen with the higher target (ratio of length of stay 1.28, 95% CI 1.09–1.50, p = 0.003) [23].
This pilot RCT was conducted to determine the safety and feasibility of a definitive multicentre RCT comparing current liberal targets for peripheral oxygen saturation with more conservative targets in critically ill children. The pilot RCT had the following objectives: (1) to test the willingness of clinicians to screen, recruit and randomise eligible patients; (2) to estimate the recruitment rate; (3) to test the acceptability of the deferred consenting procedures and participant information; (4) to test, following randomisation, delivery of and adherence to, the intervention and demonstrate separation between the groups; (5) to test follow-up for the identified, potential, patient-centred primary and other important secondary outcome measures and for adverse event reporting; (6) to inform final selection of a patient-centred primary outcome measure; and (7) to estimate the characteristics of the selected patient-centred primary outcome measure to inform sample size estimation. The underlying hypothesis is that the harm of interventions to raise peripheral oxygen saturation to > 94% exceeds the benefits of these interventions.
Methods
Study design and oversight
Oxy-PICU was a pragmatic, open, multi-centre RCT in infants and children accepted for emergency admission to one of three participating PICUs. Three units were selected as representing typical configurations for UK PICUs (general or combined general and cardiac units in general academic medical centres or within stand-alone children’s hospitals).
The trial was coordinated by the Intensive Care National Audit and Research Centre Clinical Trials Unit and sponsored by Great Ormond Street Hospital for Children NHS Foundation Trust. Health Research Authority (212228) and research ethics committee (16/SC/0617) approval were obtained. The trial was registered prior to recruitment of the first patient on ClinicalTrials.gov (NCT03040570) and the protocol published [24]. A trial steering committee, with a majority of independent members, and an independent data monitoring and ethics committee were convened to oversee the pilot trial on behalf of the sponsor.
A detailed description of the methods, including the study protocol, has been published previously [24].
Trial population and eligibility criteria
Inclusion criteria were: more than 38 weeks corrected gestational age and less than 16 years of age; within the first 6 h of face-to-face contact with PICU staff or transport team; an emergency admission accepted to a participating PICU requiring invasive or non-invasive respiratory support (including: invasive mechanical ventilation, non-invasive ventilation or high-flow humidified oxygen therapy), and receiving supplemental oxygen for abnormal gas exchange.
Mechanical ventilation was considered to include invasive and non-invasive ventilation and high-flow humidified oxygen.
Exclusion criteria were: death perceived as imminent; brain pathology/injury as primary reason for admission (e.g. traumatic brain injury, post-cardiac arrest, stroke, convulsive status epilepticus); known pulmonary hypertension; known or suspected sickle cell disease; known or suspected uncorrected congenital cardiac disease; end-of-life care plan in place with limitation of resuscitation; receiving long-term mechanical ventilation prior to this admission; or recruited to Oxy-PICU in a previous admission.
Screening and randomisation
Potentially eligible infants and children were screened against the inclusion/exclusion criteria by the transport team or PICU staff. Randomisation took place as soon as eligibility was confirmed including during transport. Participants were randomly allocated (1:1) via a secure web-based system to either the conservative (88–92%) or liberal (> 94%) peripheral oxygen saturation (SpO2) target group by a computer-generated dynamic procedure (minimisation) with a random component (80% chance of allocation to the group that minimises imbalance). Minimisation was performed on: age (< 12 months vs. ≥ 12 months); study site; primary reason for admission (lower respiratory tract infection vs. ‘other’); and severity of abnormality of gas exchange (saturation to fraction of inspired oxygen ratio < 221 with PEEP/CPAP > 5 cm H2O vs. other).
Trial interventions
Participants received supplemental oxygen and ventilator settings at the discretion of the treating clinical team with the aim of maintaining SpO2 > 94% in the liberal oxygenation group and between 88 and 92% (inclusive) in the conservative oxygen group until respiratory support was discontinued during the PICU admission. All other care was determined by the clinical team primarily responsible for the participant’s care. Data on oxygenation parameters and ventilator settings were recorded hourly from randomisation to 24 h, every 4 h from 24 to 120 h (day 6) and every 12 h from day 6 to the end of ventilation.
Consent procedures
We employed a ‘research without prior consent’ approach as is appropriate in emergency situations where any delay in commencing treatment allocation may be detrimental and when the treatments being compared are within the range of normal practice. A member of the research team approached the parents/legal representatives as soon as practical after randomisation to discuss the study, to provide a participant information sheet (Supplementary Material) and to seek consent for continued inclusion in the study. If the participant was discharged or died prior to their parents/legal representatives being approached, then they were approached by an appropriate team member at a later point either in person or by post with an option to opt out from the study at this point. The acceptability of this consent process was assessed with a 12-question multiple-choice questionnaire provided to parents/legal representatives following the approach for consent (Supplementary Material).
Outcome measures and statistics
As it was a pilot RCT, no formal sample size calculations were performed, instead a sample size of 120 was determined to be adequate to estimate candidate patient-centred outcome measures to a necessary degree of precision and to test the trial processes.
The following outcome measures were used to assess the specified objectives. Objective 1: the proportion of eligible patients recruited (target 50%), and distribution of time to randomisation. Objective 2: the number of eligible patients recruited per month. Objective 3: the proportion of parents/legal representatives refusing deferred consent. Objective 4: total time in SpO2 target range and percentage of time in range, time-weighted mean SpO2, and time-weighted mean FiO2. Objectives 5–7: characteristics and completeness of potential primary endpoints for a definitive study, including: length of PICU stay, length of invasive ventilation, ventilator-free days at day 30, duration of organ support, and PICU mortality; and observed serious adverse events.
All analyses were carried out on an intention to treat basis. Continuous variables were summarised as mean (standard deviation) and median (interquartile range), while categorical variables were summarised as number (percentage).
Results
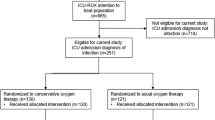
Between April and July 2017, 332 patients were screened and deemed to meet the inclusion criteria. Of these, 170 patients met one or more of the exclusion criteria and a further 40 were deemed eligible but were not randomised. Overall, 122 were randomised to the pilot RCT (Fig. 1). Three patients were randomised in error. Two were immediately removed from the study before receiving the intervention (one outside of age range, one randomised previously). One was removed from the study within hours after being recognised as meeting the ‘brain pathology as the main precipitant to admission’ exclusion criterion. Therefore, 74.8% (119/159) of eligible patients were appropriately randomised at a recruitment rate of 10 patients per month per site. Time to randomisation from first contact was a mean (SD) of 1.9 (2.2) h. Baseline characteristics were balanced between the arms (Table 1). Consent for continuation in the study was subsequently declined in 9/119 patients (7.6%). Eight of these cases were at a single study site. Three further cases (3/119, 2.5%) did not continue in the study because we were unable to seek consent (a suitable translator was not available for one family and two children were subject to care orders). Forty-four (44/116 38%) completed consent questionnaires were returned, 40/44 (91%) reported being satisfied with the consent process, while the remainder (4/44, 9%) were neither satisfied nor dissatisfied (Table 2). Full survey results including free text comments are available in the supplementary material (Tables S2 and S3). A total of 107 out of the 119 (89.9%) correctly randomised patients were therefore analysed to evaluate objectives 4–7.
The median (IQR) of time-weighted individual mean SpO2 was 94.9% (92.6–97.1) in the conservative group and 97.5% (96.2–98.4) in the liberal group (difference in medians of 2.7, 95% CI 1.3–4.0%, p < 0.001) for the full duration of mechanical ventilation. Values for the first 24 h were similar: conservative 94.7% (93–97) versus liberal 97.4% (96.3–98.2), (difference 2.8, 95% CI 1.5–4.0, p < 0.001) (Fig. 2). The median (IQR) time-weighted individual FiO2 was 0.28 (0.24–0.37) in the conservative group and 0.37 (0.30–0.42) in the liberal group (difference 0.08, 95% CI 0.03–0.13, p < 0.001) for the full duration of mechanical ventilation. The median (IQR) FiO2 values in the first 24 h were slightly higher: conservative 0.29 (0.25–0.37) and liberal 0.40 (0.31–0.5), (difference 0.11, 95% CI 0.05–0.17, p < 0.001).
Distribution of SpO2 and FiO2 by treatment group. The percentage of time at each SpO2 over the PICU stay (a, c) and median (IQR) SpO2 (b, d) and FiO2 (e) measurements at individual timepoints for the first 7 days following randomisation are shown. a, b, e Show all mechanically ventilated timepoints whereas c, d show only SpO2 values in children mechanically ventilated with FiO2 > 0.21. Shaded areas illustrate the treatment group target SpO2 ranges
Of all recorded FiO2 values, 36.4% (804/2210) were 0.21 in the conservative group compared to 13.0% (306/2347) in the liberal group (Chi-squared p < 0.001). In the first 24 h following contact with PICU or the transport team, study participants in the conservative oxygenation group spent a median (IQR) of 4.5 (1.0–10) h in the target range compared to 22 (19–23) h in the liberal group.
Candidate patient-centred outcomes had high completion rates and were similar between groups. Detailed characteristics are provided in Table 3. Two pre-specified serious adverse events (cardiac arrests) occurred, both in the liberal oxygenation group.
Discussion
In this multiple centre, parallel-group, pilot RCT, we investigated the feasibility of conducting a large-scale trial comparing conservative oxygenation (SpO2 88–92%) with liberal oxygenation (SpO2 > 94%) in critically ill children receiving respiratory support.
We observed that the eligibility criteria were effective in identifying patients and that clinicians were prepared to randomise these patients. Our initial estimates of the number of emergency admissions who met these criteria were shown to be very conservative, with recruitment being completed in approximately two-thirds of the planned study time. Indeed, our study may have further underestimated the true potential recruitment rate because the study period did not include any winter months during which admissions in acute respiratory failure predominate. The randomisation processes were timely and effective with short intervals between first contact and randomisation. The high rates of recruitment of eligible patients and low rates of consent being declined are comparable with other emergency studies in critically ill children. Families’ feedback of the consent process was both overwhelmingly supportive and in line with our recent findings in the Fluids in Shock study [25]. The variability in consent rates by institution will inform our site training for approaching families in a larger study.
The protocol achieved separation of the groups in terms of SpO2 and FiO2 values. Our SpO2 values were almost identical both in values and separations to those achieved in the CLOSE study reported by the ANZIC group in critically ill adults [18]. However, adherence to the oxygenation target range was poor in the conservative group. This was especially clear in the first few hours after randomisation. This may reflect the lack of an option to reduce FiO2 below 0.4 on many paediatric transport ventilators. In addition, many children clinically improved rapidly so that an SpO2 goal of 88–92% was not achievable because they were already breathing air. It may also be true that some bedside staff preferred values of 100% based on previous usual practice, especially during episodes of chest physiotherapy or other procedures. The pre-randomisation baseline SpO2 values of 99% reinforce the extent to which normal practice is for very liberal SpO2 values.
Further work with high-resolution (q5 s) analysis of SpO2 data is in progress to understand this behaviour, with a view to further refining the protocol. A higher baseline threshold FiO2 for inclusion (e.g. > 0.50) and a recommendation for setting an upper SpO2 alarm limit are simple protocol refinements that may further improve adherence without a major impact on the other trial processes.
Since this study was planned, a number of trials of oxygenation strategies in adults have reported or opened for recruitment. The HYPER2S trial in adults with septic shock employed a factorial design comparing an FiO2 1.0 with an SpO2 target of 88–95% alongside hypertonic versus isotonic volume resuscitation. It was stopped prematurely for safety concerns of increased weakness and atelectasis in the hyperoxia group with a trend to increased mortality [26]. The single-centre Oxygen-ICU study observed reduced mortality with modestly reduced oxygenation targets (SpO2 94–98 vs. 97–100%) [19]. A number of larger studies are currently recruiting, including: evaluating the effects of two approaches to oxygen therapy in intensive care unit patients requiring life support (ICU-ROx), ACTRN 12615000957594 by the ANZICS group, and handling oxygenation targets in the intensive care unit (HOT-ICU) in Denmark, NCT03174002; optimal oxygenation in the intensive care unit (O2-ICU) in the Netherlands, NCT02321072; Liberal Oxygenation Versus Conservative Oxygenation in ARDS (LOCO2) in France NCT02713451 and Targeted Oxygen therapy in Critical illness (TOXYC) NCT03287466 in the UK. Maitland and colleagues are conducting a large study of oxygen treatment thresholds combined with a comparison of high flow versus low flow oxygen delivery in east Africa, ISRCTN15622505 [27].
Our study shares a number of weaknesses with the majority of these trials. Exclusion of cases with acute encephalopathy or congenital cardiac disease limits the generalisability of any findings to a subset of critically ill children. These were felt to be necessary because of our work scoping current practice and equipoise [7]. We did not attempt to control oxygen therapy prior to PICU referral. We did not attempt to blind clinical staff to the group allocation. Our pragmatic approach means that clinicians were free to adopt different haemodynamic goals, temperature control strategies or transfusion thresholds that might alter the balance between oxygen delivery and consumption independent of the SpO2 targets. These multiple interactions may only be tractable with more complex adaptive trial designs [28]. In addition, as a feasibility study, we cannot make any conclusions on the effectiveness of conservative oxygenation.
There are also several strengths of Oxy-PICU beyond it being the first report of a randomised comparison of conservative and liberal oxygenation strategies in critically ill children. We have demonstrated a high degree of engagement of clinical staff with the protocol across different units and transport teams. The trial processes were acceptable to parents/legal representatives. No safety issues were identified and there were trends across the clinical outcomes for shorter durations of organ support in the conservative group that might be suitable as outcome measures in a full trial.
Although the choice of primary outcome measure for a definitive trial will involve consultation with patients and families and considerations of cost, timings and competing studies, our data permit sample size estimations. For example, a ‘ventilator-free days’ outcome with 90% power to detect a 1.25-day difference in ventilation and no effect on mortality would require a total of 2014 patients. These pilot data support the feasibility of a trial on this scale in critically-ill children.
Conclusion
This Oxy-PICU study has demonstrated that it is feasible to conduct a large pragmatic clinical trial of conservative versus liberal oxygenation in critically ill children. Considerations for a full trial include addition of an FiO2 threshold for inclusion, and a recommendation for an upper SpO2 alarm limit in the conservative group.
References
Vincent JL, De Backer D (2013) Circulatory shock. N Engl J Med 369:1726–1734. https://doi.org/10.1056/NEJMra1208943
Asfar P, Singer M, Radermacher P (2015) Understanding the benefits and harms of oxygen therapy. Intensive Care Med 41:1–4. https://doi.org/10.1007/s00134-015-3670-z
Martin DS, Grocott MPW (2013) Oxygen therapy in critical illness. Crit Care Med 41:423–432. https://doi.org/10.1097/CCM.0b013e31826a44f6
PICANet (2017) Paediatric Intensive Care Audit Network, pp1–51. https://www.picanet.org.uk/Audit/Annual-Reporting/PICANet_2017_Annual_Report_Tables_and_Figures_FINAL_v2.0.pdf
Helmerhorst HJ, Schultz MJ, van der Voort PH et al (2014) Self-reported attitudes versus actual practice of oxygen therapy by ICU physicians and nurses. Ann Intensive Care 4:23. https://doi.org/10.1186/s13613-014-0023-y
de Graaff AE, Dongelmans DA, Binnekade JM, de Jonge E (2011) Clinicians’ response to hyperoxia in ventilated patients in a Dutch ICU depends on the level of FiO2. Intensive Care Med 37:46–51. https://doi.org/10.1007/s00134-010-2025-z
Raman S, Ray S, Peters MJ (2016) Survey of oxygen delivery practices in UK paediatric intensive care units. Crit Care Res Pract 2016:1–4. https://doi.org/10.1155/2016/6312970
Ray S, Rogers L, Raman S, Peters MJ (2016) Liberal oxygenation in paediatric intensive care: retrospective analysis of high-resolution SpO2 data. Intensive Care Med 43:146–147. https://doi.org/10.1007/s00134-016-4606-y
Elmer J, Scutella M, Pullalarevu R et al (2015) The association between hyperoxia and patient outcomes after cardiac arrest: analysis of a high-resolution database. Intensive Care Med 41:49–57. https://doi.org/10.1007/s00134-014-3555-6
Kilgannon JH, Jones AE, Parrillo JE et al (2011) Relationship between supranormal oxygen tension and outcome after resuscitation from cardiac arrest. Circulation 123:2717–2722. https://doi.org/10.1161/circulationaha.110.001016
Sznycer-Taub NR, Lowery R, Yu S et al (2016) Hyperoxia is associated with poor outcomes in pediatric cardiac patients supported on venoarterial extracorporeal membrane oxygenation. Pediatr Crit Care Med 17:350–358. https://doi.org/10.1097/PCC.0000000000000655
Rincon F, Kang J, Maltenfort M et al (2014) Association between hyperoxia and mortality after stroke: a multicenter cohort study. Crit Care Med 42:387–396. https://doi.org/10.1097/CCM.0b013e3182a27732
de Jonge E, Peelen L, Keijzers PJ et al (2008) Association between administered oxygen, arterial partial oxygen pressure and mortality in mechanically ventilated intensive care unit patients. Crit Care 12:R156. https://doi.org/10.1186/cc7150
Raman S, Prince NJ, Hoskote A et al (2016) Admission PaO2 and mortality in critically ill children: a cohort study and systematic review. Pediatr Crit Care Med 17:e444–e450. https://doi.org/10.1097/PCC.0000000000000905
Helmerhorst HJF, Schultz MJ, van der Voort PHJ et al (2015) Bench-to-bedside review: the effects of hyperoxia during critical illness. Crit Care 19:1–12. https://doi.org/10.1186/s13054-015-0996-4
SUPPORT Study Group of the Eunice Kennedy Shriver, NICHD Neonatal Research Network, Carlo WA, Finer NN et al (2010) Target ranges of oxygen saturation in extremely preterm infants. N Engl J Med 362:1959–1969. https://doi.org/10.1056/nejmoa0911781
BOOST II United Kingdom Collaborative Group, BOOST II Australia Collaborative Group, BOOST II New Zealand Collaborative Group et al (2013) Oxygen saturation and outcomes in preterm infants. N Engl J Med 368:2094–2104. https://doi.org/10.1056/nejmoa1302298
Panwar R, Hardie M, Bellomo R et al (2015) Conservative versus liberal oxygenation targets for mechanically ventilated patients—a pilot multicenter randomized controlled trial. Am J Respir Crit Care Med 193(1):43–51. https://doi.org/10.1164/rccm.201505-1019OC
Girardis M, Busani S, Damiani E et al (2016) Effect of conservative vs conventional oxygen therapy on mortality among patients in an intensive care unit. JAMA 316:1–7. https://doi.org/10.1001/jama.2016.11993
Khoshnood A, Carlsson M, Akbarzadeh M et al (2015) The effects of oxygen therapy on myocardial salvage in ST elevation myocardial infarction treated with acute percutaneous coronary intervention: the supplemental oxygen in catheterized coronary emergency reperfusion (SOCCER) study. Cardiology 132:16–21. https://doi.org/10.1159/000398786
Stub D, Smith K, Bernard S et al (2015) Air versus oxygen in ST-segment-elevation myocardial infarction. Circulation 131:2143–2150. https://doi.org/10.1161/circulationaha.114.014494
Hofmann R, James SK, Jernberg T et al (2017) Oxygen therapy in suspected acute myocardial infarction. N Engl J Med 377:1240–1249. https://doi.org/10.1056/nejmoa1706222
Cunningham S, Rodriguez A, Adams T et al (2015) Oxygen saturation targets in infants with bronchiolitis (BIDS): a double-blind, randomised, equivalence trial. Lancet 386:1041–1048. https://doi.org/10.1016/S0140-6736(15)00163-4
Jones GAL, Ramnarayan P, Raman S et al (2017) Protocol for a randomised pilot multiple centre trial of conservative versus liberal oxygenation targets in critically ill children (Oxy-PICU). BMJ Open 7:e019253–e019258. https://doi.org/10.1136/bmjopen-2017-019253
O’Hara CB, Canter RR, Mouncey PR et al (2017) A qualitative feasibility study to inform a randomised controlled trial of fluid bolus therapy in septic shock. Arch Dis Child 103(1):28–32. https://doi.org/10.1136/archdischild-2016-312515
Asfar P, Schortgen F, Boisramé-Helms J et al (2017) Hyperoxia and hypertonic saline in patients with septic shock (HYPERS2S): a two-by-two factorial, multicentre, randomised, clinical trial. Lancet Respir Med 5:180–190. https://doi.org/10.1016/S2213-2600(17)30046-2
Maitland K, Kiguli S, Opoka RO et al (2017) Children’s oxygen administration strategies trial (COAST): a randomised controlled trial of high flow versus oxygen versus control in African children with severe pneumonia. Wellcome Open Res 2:100. https://doi.org/10.12688/wellcomeopenres.12747.1
Bhatt DL, Mehta C (2016) Adaptive designs for clinical trials. N Engl J Med 375:65–74. https://doi.org/10.1056/NEJMra1510061
Acknowledgements
The authors would like to thank the medical and nursing staff at each trial site, the Paediatric Intensive Care Society Study Group, and the patients and their families who contributed to this study. We are also grateful to members of the Trial Steering Committee and Data Monitoring Committee for their assistance in performing this study.
Funding
This study was funded by Great Ormond Street Hospital Children’s Charity. Registered Charity No. 235825 and supported by the National Institute for Health Research Great Ormond Street Hospital Biomedical Research Centre. The views expressed are those of the authors and not necessarily those of the NHS, the NIHR or the UK Department of Health.
Author information
Authors and Affiliations
Consortia
Corresponding author
Ethics declarations
Conflicts of interest
No conflicts of interest to declare.
Additional information
The Oxy-PICU trial has been registered at ClinicalTrials.gov (NCT03040570).
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Peters, M.J., Jones, G.A.L., Wiley, D. et al. Conservative versus liberal oxygenation targets in critically ill children: the randomised multiple-centre pilot Oxy-PICU trial. Intensive Care Med 44, 1240–1248 (2018). https://doi.org/10.1007/s00134-018-5232-7
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00134-018-5232-7