Abstract
Summary
Denosumab and zoledronic acid are potent antiresorptives. In this study in patients pre-treated with zoledronic acid, denosumab achieved similar increases with zoledronic acid in lumbar spine BMD despite the more prominent reduction of bone turnover markers. Denosumab reversibly reduced endogenous RANKL.
Introduction
We aimed to compare yearly changes in lumbar spine (LS) bone mineral density (BMD), bone turnover markers, free soluble receptor activator of nuclear factor kappaB ligand (sRANKL) and sclerostin levels between denosumab and zoledronic acid.
Methods
Postmenopausal women with low bone mass previously treated with zoledronic acid for 1 year were assigned to denosumab injection (n = 32) or zoledronic acid infusion (n = 26). Procollagen type 1 N-terminal propeptide (P1NP), C-terminal cross-linking telopeptide of type 1 collagen (CTx), sRANKL, and sclerostin levels were measured in serum samples obtained at baseline and 3, 6, and 12 months after denosumab injection or zoledronic acid infusion. LS BMD was measured at baseline and 12 months.
Results
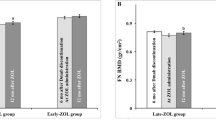
The mean LS increase was 4.5 and 4.4 % with denosumab and zoledronic acid, respectively (p = 0.560). Denosumab caused a larger decrease in CTx at 3 months (p < 0.001) and P1NP at 3 (p < 0.001), 6 (p = 0.021), and 12 months (p = 0.042). Denosumab significantly decreased sRANKL by 87.4 % at 3 months (p < 0.001). Sclerostin levels were not changed with either intervention (p = 0.162 and p = 0.214, respectively).
Conclusions
In patients previously treated with zoledronic acid, denosumab reduces bone turnover more than zoledronic acid, but the increases in LS BMD are comparable. Furthermore, denosumab administration results in reversible inhibition of the metabolically significant endogenous free soluble RANKL levels. Serum sclerostin is not affected by either agent.


Similar content being viewed by others
References
Nancollas GH, Tang R, Phipps RJ, Henneman Z, Gulde S, Wu W, Mangood A, Russell RG, Ebetino FH (2006) Novel insights into actions of bisphosphonates on bone: differences in interactions with hydroxyapatite. Bone 38:617–627
Cummings SR, San Martin J, McClung MR et al (2009) Denosumab for prevention of fractures in postmenopausal women with osteoporosis. N Engl J Med 361:756–765
Black DM, Delmas PD, Eastell R et al (2007) Once-yearly zoledronic acid for treatment of postmenopausal osteoporosis. N Engl J Med 356:1809–1822
Stolina M, Dwyer D, Middleton S, Adamu S, Ominsky MS, Zack D, Kostenuik PJ (2007) OPG prevents osteopenia in arthritic rats via different mechanisms than bisphosphonate therapy. J Bone Miner Res 22(Suppl 1):S475
Baron R, Ferrari S, Russell RG (2011) Denosumab and bisphosphonates: different mechanisms of action and effects. Bone 48:677–692
Brown JP, Prince RL, Deal C et al (2009) Comparison of the effect of denosumab and alendronate on BMD and biochemical markers of bone turnover in postmenopausal women with low bone mass: a randomized, blinded, phase 3 trial. J Bone Miner Res 24:153–161
Kendler DL, Roux C, Benhamou CL, Brown JP, Lillestol M, Siddhanti S, Man HS, San Martin J, Bone HG (2010) Effects of denosumab on bone mineral density and bone turnover in postmenopausal women transitioning from alendronate therapy. J Bone Miner Res 25:72–81
Recknor C, Czerwinski E, Bone HG et al (2013) Denosumab compared with ibandronate in postmenopausal women previously treated with bisphosphonate therapy: a randomized open-label trial. Obstet Gynecol 121:1291–1299
Roux C, Hofbauer LC, Ho PR et al (2014) Denosumab compared with risedronate in postmenopausal women suboptimally adherent to alendronate therapy: efficacy and safety results from a randomized open-label study. Bone 58:48–54
Brown JP, Roux C, Ho PR et al (2014) Denosumab significantly increases bone mineral density and reduces bone turnover compared with monthly oral ibandronate and risedronate in postmenopausal women who remained at higher risk for fracture despite previous suboptimal treatment with an oral bisphosphonate. Osteoporos Int 25:1953–1961
Mandema JW, Zheng J, Libanati C, Perez Ruixo JJ (2014) Time course of bone mineral density changes with denosumab compared with other drugs in postmenopausal osteoporosis: a dose-response-based meta-analysis. J Clin Endocrinol Metab 99:3746–3755
Anastasilakis AD, Polyzos SA, Gkiomisi A, Bisbinas I, Gerou S, Makras P (2013) Comparative effect of zoledronic acid versus denosumab on serum sclerostin and dickkopf-1 levels of naïve postmenopausal women with low bone mass: a randomized, head-to-head clinical trial. J Clin Endocrinol Metab 98:3206–3212
Eastell R, Christiansen C, Grauer A et al (2011) Effects of denosumab on bone turnover markers in postmenopausal osteoporosis. J Bone Miner Res 26:530–537
Papapoulos S, Chapurlat R, Libanati C et al (2012) Five years of denosumab exposure in women with postmenopausal osteoporosis: results from the first 2 years of the FREEDOM extension. J Bone Miner Res 27:694–701
Black DM, Reid IR, Boonen S et al (2012) The effect of 3 versus 6 years of zoledronic acid treatment of osteoporosis: a randomized extension to the horizon-pivotal fracture trial (PFT). J Bone Miner Res 27:243–254
Bone HG, Chapurlat R, Brandi ML et al (2013) The effect of 3 or 6 years of denosumab exposure in women with postmenopausal osteoporosis: results from the FREEDOM extension. J Clin Endocrinol Metab 98:4483–4492
McClung M, Recker R, Miller P, Fiske D, Minkoff J, Kriegman A, Zhou W, Adera M, Davis J (2007) Intravenous zoledronic acid 5 mg in the treatment of postmenopausal women with low bone density previously treated with alendronate. Bone 41:122–128
Bone HG, Bolognese MA, Yuen CK, Kendler DL, Miller PD, Yang YC, Grazette L, San Martin J, Gallagher JC (2011) Effects of denosumab treatment and discontinuation on bone mineral density and bone turnover markers in postmenopausal women with low bone mass. J Clin Endocrinol Metab 96:972–980
Polyzos SA, Anastasilakis AD, Efstathiadou Z et al (2009) The effect of zoledronic acid on serum dickkopf-1, osteoprotegerin, and RANKL in patients with Paget’s disease of bone. Horm Metab Res 41:846–850
Çankaya M, Cizmeci Şenel F, Kadioglu Duman M, Muci E, Dayisoylu EH, Balaban F (2013) The effects of chronic zoledronate usage on the jaw and long bones evaluated using RANKL and osteoprotegerin levels in an animal model. Int J Oral Maxillofac Surg 42:1134–1139
Mercatali L, Ricci M, Scarpi E et al (2013) RANK/RANK-L/OPG in patients with bone metastases treated with anticancer agents and zoledronic acid: a prospective study. Int J Mol Sci 14:10683–10693
Polyzos SA, Anastasilakis AD, Terpos E (2009) Transient secondary hyperparathyroidism following intravenous infusion of zoledronic acid. Support Care Cancer 17:1329–1330
Makras P, Polyzos SA, Papatheodorou A, Kokkoris P, Chatzifotiadis D, Anastasilakis AD (2013) Parathyroid hormone changes following denosumab treatment in postmenopausal osteoporosis. Clin Endocrinol (Oxf) 79:499–503
Acknowledgments
We thank Roche Diagnostics Hellas for providing us the kits for the measurements of CTx, P1NP, PTH, and 25(OH)D.
Conflicts of interest
Athanasios D. Anastasilakis has received lecture fees and research grant from Amgen and lecture fees from Lilly; Stergios A. Polyzos has received lecture fee and research grant from Amgen; Athina Gkiomisi, Zacharias G. Saridakis, Dimitrios Digkas, Ilias Bisbinas, Grigorios T. Sakellariou, Athanasios Papatheodorou, and Panagiotis Kokkoris have nothing to declare. Polyzois Makras has received lecture fees and research grants from Amgen, and lecture fees from Glaxo, Lilly, Pfizer, Leo, Genesis, ELPEN, VIANEX. Neither Amgen nor Novartis had any implication in any stage of this study (study’s conception and design, analysis and interpretation of data, drafting, or revising the manuscript).
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Anastasilakis, A.D., Polyzos, S.A., Gkiomisi, A. et al. Denosumab versus zoledronic acid in patients previously treated with zoledronic acid. Osteoporos Int 26, 2521–2527 (2015). https://doi.org/10.1007/s00198-015-3174-2
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00198-015-3174-2