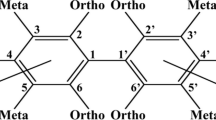
Abstract
Experimental reproductive and developmental toxicity studies with polychlorinated biphenyls (PCBs) are reviewed in brief to determine their relevance for current environmental exposure of humans during the prenatal and postnatal developmental periods. Additional material is published in electronic form only, which contains graphic overviews on individual PCBs and various mixtures that are linked with the relevant citations. In this comprehensive article we focus on interactions of PCBs with biological substrates that could mediate adverse effects observed in experimental animals and in children, and the shortcomings of many of the animal studies available. A main point of criticism involves the relative lack of animal data on several of those persistent congeners, either as individual compounds or as environmentally relevant mixtures, which are currently used as a measure of human exposure. Experimental studies in animals are frequently conducted with commercial PCB mixtures, a test design that does not reflect the exposure situation in humans. Important improvements of animal experiments could be achieved by more complete reporting of litter data (pre- and post-natal losses, toxic signs in the dam and the offspring, birth weights and postnatal growth data), the inclusion of endpoints that have been found previously to be affected by PCBs, and measurements of internal exposure data.
Similar content being viewed by others
The magnitude of the problem
PCB levels in human populations
Twenty years after their production ban in most industrialised countries, PCBs are still present in the environment and in the food chain. Exposure in humans occurs mainly from this environmental contamination of food products. In addition, children are exposed to part of the body burden of their mothers during intrauterine development and through breast-feeding after birth. Several epidemiological studies in different parts of the world have tried to analyse possible adverse effects of this PCB exposure on neurodevelopment of children. Exposure levels in the collective studies ranged from very high and overtly toxic to low environmental levels. Reviews of these studies come to somewhat different conclusions. While Kimbrough et al. (2001) could see no conclusive evidence that environmental exposure to PCBs affects the neurobehavioral development of infants and children, Ribas-Fito and co-workers concluded that these studies suggest a subtle adverse effect of prenatal PCB exposure on child neurodevelopment, but do not allow the derivation of the degree of risk associated with current levels of exposure (Ribas-Fito et al. 2001). Schantz and coworkers concluded that there is growing evidence for PCB effects on neurodevelopment in humans, but also pointed out that many of the PCB congeners present in human tissues have never been studied in the laboratory, and their relative potency to produce nervous system effects is unknown (Schantz et al. 2003).
Concentrations measured in human populations differ depending on the age of the subjects, their nutrition, the origin of the samples and other factors. For detailed data of this aspect the reader is referred to the specific literature. In general, measurements of plasma and tissue levels in humans have indicated that exposure to these chemicals is on the decline since the production of PCBs has been significantly reduced. The three persistent PCBs measured in these studies, 2,2′,3′,4,4′,5-hexachlorobiphenyl (PCB 138), 2,2′,4,4′,5,5′-hexachloro-biphenyl (PCB 153) and 2,2′,3,4,4′,5,5′-heptachlorobiphenyl (PCB 180), are the most abundant in biological extracts. However, minor components may also be important because of their toxic potency. Mean concentrations of total PCBs in young humans (neonates to 25 years of age) are reported in the current literature to be in the range 0.56–1.2 ng/ml serum or approximately 150–200 ng/g lipid weight (Umweltbundesamt 1999, 2002; Becker et al. 2002; Lackmann 2002; Nawrot et al. 2002; Voorspoels et al. 2002).
Many experimental studies using commercial mixtures have been published to clarify the possible risk to humans who are mainly exposed via food. However, the exposure conditions for humans who are confronted with a mixture of persistent PCB congeners that have already been filtered through the ecosystem are very different from most experimental study situations in which animals are exposed to single congeners or commercially available mixtures of PCBs. Thus, data on single PCB congeners can considered to be relevant when the respective substance persists in the environment, whereas commercial mixtures (Aroclors, Clophens) are not truly representative of the PCB exposure encountered by humans today. Some authors, therefore, have used reconstituted PCB mixtures with compositions similar to the congener range found in human milk.
Animal studies have mainly been conducted in rodents (rats, mice). A few non-human primate collectives have also been studied, the results being published in several series of papers (Allen and Barsotti 1976; Arnold et al. 1993a, 1993b, 1997, 1999; Barsotti and Van Miller 1984; Levin et al. 1988; Mele et al. 1986; Mes et al. 1994; Rice 1999b; Rice and Hayward 1997; Schantz et al. 1989, 1991; Tryphonas et al. 1991). Although rhesus and cynomolgus monkeys can be considered good model species for humans, especially for the complex behavioural aspects, all monkey studies published so far have considerable drawbacks. For example, they used dosages that elicited obvious PCB toxicity (low pregnancy rate, infant death, skin lesions, hyperpigmentation) in either mother or offspring and thus are representative of highly exposed human populations only. In addition, the low numbers of offspring evaluated (usually below ten per group) and the use of feral monkeys with unknown age and reproductive history does not inspire great confidence in the results of these studies. We decided therefore not to include these studies. In this review article we briefly summarise the available experimental data on developmental toxicity of PCBs in rodents. Results with selected congeners are shown in Figs. 1, 2 and 3; for an overview see Fig. 4 and Table 2.
Three studies in two different rat strains were evaluated, two with prenatal and one with postnatal exposure of the dam. Reference numbers in square brackets, as well as strain, exposure period, daily dose and cumulative dose (in brackets) are listed on the left side of the figure. Brain PCB levels were measured only in the postnatal exposure study. Colored arrows indicate the tested parameters that were affected (m = male, f = female). The line ending in a bar (no arrow head) indicates that grip strength was unaffected by postnatal exposure through the milk. The most sensitive parameters in this set of studies (affected at the lowest dose tested) are marked with red dots
Fourteen studies in three different strains of rats (SD = Sprague-Dawley, LE = Long-Evans, Wistar Rats) were evaluated, all of them with exposure through the mother animal. Reference numbers in square brackets, as well as strain, exposure period, daily dose and cumulative dose (in brackets) are listed on the left side of the figure. Colored arrows indicate the tested parameters that were affected (m = male, f = female). The most sensitive parameters in this set of studies (affected at the lowest dose tested) are marked with red dots. Exposure to 3 mg/kg/day during the fetal period reduced pup viability (dose field marked in red). All other dose regimens were compatible with postnatal survival of the offspring. Two studies provided PCB brain levels and one study found hydroxylated metabolites as the main PCB compounds in the fetuses. Growth reduction, delayed eye opening, alterations in anogenital distance in ales (decrease) and females (increase), increased sperm counts, decreased sexual receptivety in females and in an increase in brain dopamine/dopamine turnover were the most sensitive parameters in different studies
Six studies in three different strains of mice (B6 = C57BL/6) were evaluated, two from them with direct postnatal exposure of the offspring. Reference numbers in square brackets, as well as strain, exposure period, daily dose and cumulative dose (in brackets) are listed on the left side of the figure. Colored arrows indicate the tested parameters that were affected (m = male, f = female). The most sensitive parameters in this set of studies (affected at the lowest dose tested) are marked with red dots. Dose levels of 8 mg/kg or higher were overtly toxic for the conceptus of the offspring (dose field marked in red) and therefore cannot be considered relevant for current human exposure. This induces the only study where behavioral parameters were tested after exposure in utero. No PCB tissue levels were available for any of the studies. NMRI mouse pups treated with a single low dose postnatally displayed a decrease of muscarinic receptor binding in the hippocampus in the preweaning period and an altered motor activity pattern when tested in the open field as adults. This was characterized by decreased initial exloratory activity and a lack of habituation over time. Publications considered are: [1] Agrawal et al. (1981); [2] d’Argy et al. (1987); [3] Marks et al. (1989); [4] Eriksson (1988); [5] Eriksson (1991); [6] Esser et al. (1994)
Changes in behavioral parameters, brain biochemistry or electrophysiology have been observed for most of the PCB congeners and mixtures evaluated in this review. However, not all of the effects were detrimental. Spatial memory was found to be improved in rats that were exposed to the coplanar congeners 77 and 126 during devlopment. The learning impairment observed with other PCBs appears unrelated to the decrease in circulating thyroid hormone as it also appeared when plasma T4 was unaffected (PCB 28). Overall, the data available for individual compounds and mixtures are insufficient to derive patterns of action on the brain and the resulting behavioral changes. For references and more details see Table 2
The amount of literature on PCB-induced developmental effects in rodents is enormous, and it did not seem possible to describe all papers in detail in this review without impairing the readability. Therefore, in addition to the printed comprehensive version of the review, data with other congeners and mixtures are summarised in a series of similarly designed figures, which are available as additional electronic material in the online version of this article at http://dx.doi.org/10.1007/s00204-003-0519-y; this material contains graphic overviews on different PCBs, which are linked with the relevant citations. All compounds and mixtures which are subject of the review and the supplementary electronic material are listed in Table 1.
Hormonal interactions
Although only relatively few of the 209 theoretically possible PCB congeners persist in the environment, they have been found to interact with a multitude of systems, probably mostly in the form of their hydroxylated metabolites. Of special interest are interactions of PCBs and/or their metabolites with hormonal systems, which themselves influence a variety of processes. PCBs may act through their structural similarities to thyroid hormones and other nuclear receptor ligands, through binding to the arylhydrocarbon receptor (AhR) by inducing AhR-dependent genes, and through the induction or inhibition of various metabolic enzymes. Moreover, it appears highly likely that not all interactions of PCBs with developmental processes have been identified yet. Since thyroid hormones, estrogens and androgens play an important role in the development of mammals, environmental PCB exposure during sensitive developmental periods may result in adverse effects on the growth and functional integrity of the organism, especially in the brain.
Thyroid hormone depletion by PCBs
One of the most consistent effects of PCB exposure in adult and neonatal rodents is a decrease in serum thyroxine (T4) concentration. One of the mechanisms discussed for this decrease is the ability of PCBs to induce hepatic microsomal enzymes UDP-glucuronosyltransferases (UGTs), which conjugate triiodothyronine (T3) and T4 before biliary excretion. In fact, an increased glucuronidation activity for T4 has been found in hepatic microsomes from pregnant rats treated with PCB 169 and PCB 77 on day 1 of pregnancy and days 2–18, respectively, as well as in their fetuses and offspring throughout the lactation period (Morse et al. 1993). Similar results were seen in pregnant and weanling rats exposed to Aroclor 1254 (A1254) from day 10–16 of pregnancy (Morse et al. 1996). However, circulating concentrations of thyroid stimulating hormone (TSH), which controls thyroid hormone synthesis, have not been found to be increased under conditions of PCB exposure and, consistent with this finding, a much smaller decrease than the one for T4 is seen for the active hormone T3, mostly with high doses that deplete T4 severely.
T3 plasma and brain concentrations are maintained in the normal range under PCB exposure by an upregulation in the activity of the type II thyroxine 5′-deiodinase, which produces T3 from T4 (Morse et al. 1993; Morse et al. 1996). Metabolic inactivation of T3 and its excretion have not been analysed in these studies. However, data from young adult male rats treated with A1254 in the diet (100 ppm) for 7 days show that although T4 and T3 serum concentrations were decreased by 67% and 21%, respectively, there was no increase in biliary excretion of T3 metabolites (Vansell and Klaassen 2002). Therefore, any depletion of T3 in the plasma appears to be mediated by an insufficient supply of T4 for conversion and/or by an increased tissue uptake, but not by an increased loss of T3 from the body.
Adult humans exposed to PCBs through their diet were found to have decreased plasma levels of thyroid hormones (Persky et al. 2001) but since the values were well within the normal range and well above the hypothyroidal range a toxicological relevance is questionable. No relationship between cord blood T4 and PCB levels could be found in a cohort of North Carolina neonates born between 1978 and 1982 (Longnecker et al. 2000).
There is mounting evidence that PCB effects are also mediated by PCB metabolites. Methylsulfonyl metabolites of tetra- and penta-chlorinated biphenyls, the major methylsulfonyl metabolites found in human tissue and milk, have been shown to decrease T4 and T3 serum levels in adult rats (Kato et al. 1999), and hydroxylated metabolites of PCBs with the OH-group in meta or para position are potent inhibitors of thyroid hormone sulfation by enzymes of the phenol sulfotransferase family in vitro (Schuur et al. 1998a, 1998b). Unfortunately it is difficult to compare relative potencies of PCBs and their metabolites in vitro because of the extensive adsorption of these substances to the plastic wells and glassware used in such experiments (Layton et al. 2002).
Interactions with thyroid hormone binding proteins and receptors
In the plasma of rodents, T4 is bound to transthyretin (prealbumin), which in addition to thyroxine transports the complex of retinol-binding protein and retinol. The more important T4 plasma transport protein for humans, thyroxine-binding globulin (TBG), is not present in rats and mice except during the period of postnatal thyroid development until 3 weeks after birth and in the hypothyroidal state (Savu et al. 1989; Vranckx et al. 1990). Although transthyretin is only a secondary carrier of T4 in human plasma, it is the primary T4 carrier in cerebrospinal fluid (Robbins 1996; White and Kelly 2001) and is implicated in the transfer of T4 to the fetal compartment (Meerts et al. 2002). PCB congeners and their hydroxylated metabolites with structural and conformational similarities to T4 bind to rodent and human transthyretin, many with affinity similar to or higher than the endogenous ligand. Hydroxylated metabolites bind strongly to human transthyretin and can displace T4 in vitro from this protein even where the parent congener is inactive in this respect. In contrast, TBG binds most PCBs very weakly or not at all, as does the thyroid hormone receptor β. The affinity of PCB metabolites for human TBG is at least two orders of magnitude lower than that of T4 (Cheek et al. 1999; Lans et al. 1994). It would appear therefore that plasma transport of T4 in humans is less sensitive to PCB exposure than that in rodents. However, the effects on T4 supply to the brain may be similar in humans and rodents due to the involvement of transthyretin in this process.
To complicate matters further, the planar PCBs 77, 126 and 169 (or their metabolites) and commercial mixtures that contain them (A1254) seem to possess not only antithyroid but also some thyreomimetic properties in developmental studies. Rat pups exposed to these substances or to A1254 open their eyes at an earlier time-point, an effect that is also elicited with an excess of l-thyroxine (Schantz et al. 1997a; Gray et al. 1999; Goldey et al. 1995a; Brosvic et al. 2002). Treatment of rat dams with A1254 during pregnancy and lactation at doses greater than 1 mg/kg per day increased RC3/neurogranin mRNA in their pups on postnatal day 15 (PND15) and restored myelin basic protein mRNA to the normal level. A lower dose of 1 mg/kg reduced myelin basic protein mRNA compared with that in controls (Zoeller et al. 2000). These findings show that A1254 contains antagonistic as well as agonistic components that can affect the transcription of these thyroid receptor-inducible neuronal protein genes. RC3/neurogranin is implicated in long-term potentiation and learning/memory processes and appears in rat brain around prenatal day 18 with a rapid increase during the first two postnatal weeks when thyroid hormone production is upregulated (Miyakawa et al. 2001). Another indication for a thyreomimetic function of PCBs could be the selective hearing loss that can be induced with A1254 and PCB 126 (Herr et al. 2001; Crofton and Rice 1999). While propylthiouracil-induced hypothyroidism increased auditory thresholds in developing rats over a range of frequencies (1–40 kHz), only low-frequency (1 kHz) hearing ability was affected in PCB-exposed animals, despite a similar reduction in T4 serum concentrations (Goldey et al. 1995a, 1995b). Histological examinations of a few PCB-exposed rats showed a mild-to-moderate loss of outer hair cells in the upper-middle and apical turns of the cochlea. However, such changes were only observed at doses that induced pronounced postnatal mortality (Crofton et al. 2000a).
Estrogenic and antiestrogenic activity of PCBs
PCBs can induce estrogenic as well as antiestrogenic effects, and evidence is accumulating that this is due partly to the generation of hydroxylated PCB metabolites (Layton et al. 2002). Estrogenic and antiestrogenic effects observed in cell cultures (Kramer et al. 1997) seem to be restricted mostly to readily metabolised substances, which are rapidly excreted in animals and humans and do not persist in the environment. Hydroxylated metabolites of PCBs bind to estrogen receptors ERα and ERβ with similar preference, but their binding affinities are at least 1000-fold lower than that of 17β-estradiol (E2), a measure that may be confounded by the preferential adsorption of the PCB metabolites to the test vials (Layton et al. 2002). Such metabolites have been shown to compete with E2 and to act as agonists or antagonists in various models. For example, 4-hydroxy-2′,4′,6′-trichlorobiphenyl and 4-hydroxy-2′,3′,4′,5′-tetrachlorobiphenyl stimulate transcriptional activity of both ERs but compared to E2 may induce a different preference of the receptor–ligand complex for specific estrogen response elements on the DNA and the preferential binding of other cofactors (Connor et al. 1997; Kuiper et al. 1998; Hall et al. 2002).
In addition to direct interactions with ERs that misdirect their translational or inhibitory actions, other influences on estrogenic mechanisms are possible: the local production of estrogen from testosterone is catalysed by the aromatase enzyme complex. This reaction is especially important for the development of sexual dimorphic brain regions, implicated in male and female specific behaviour. Both environmentally relevant PCBs and 2,3,7,8-tetrachlorodibenzo-p-dioxin (TCDD) have been shown to reduce aromatase activity in the brain of newborn male rats (Hany et al. 1999b; Ikeda et al. 2002). The mechanisms for this reduction remain to be clarified; however, for TCDD no direct effect on enzyme activity in vitro could be detected. On the other hand, hydroxylated PCBs inhibit members of the hydroxysteroid sulfotransferase family, as has been shown with human estrogen sulfotransferase, and thus may increase availability of endogenous estrogens (Kester et al. 2000).
Antiestrogenicity of PCBs may be mediated not only by an interaction with estrogen receptors but also by cross-talk of the arylhydrocarbon receptor (AhR) with ER pathways after activation by TCDD-like PCBs. Inhibitory dioxin-responsive elements have been found in promoter regions of some E2-responsive target genes (Safe et al. 1998) and can be expected to modulate ER-directed gene expression. In addition, data from rat hepatocyte cultures indicate that the AhR activates the transcription of several other nuclear transcription factors belonging to the hepatocyte nuclear factor and the CCAAT/enhancer-binding protein families, which then can activate or repress other genes not directly responsive to the AhR (Borlak et al. 2002).
Bonefeld-Jørgensen used several in vitro systems (MCF-7 cells, Chinese hamster ovary cells) and showed that three environmentally persistent congeners possess receptor-mediated antiestrogenic (PCB 138, PCB 153 and PCB 180) and antiandrogenic (PCB 138) effects (Bonefeld-Jørgensen et al. 2001).
Androgenic and antiandrogenic activity of PCBs
In mice and rats anogenital distance (AGD) is an indicator of prenatal androgenisation. Endogenous androgen production as well as the androgen concentrations provided by adjacent littermates in the uterus play a role in determining AGD. Higher androgen concentrations are associated with longer AGDs (Vandenbergh and Huggett 1995; Hernandez-Tristan et al. 1999). Prenatal differences in androgenisation also modulate social and sexual behaviour in these species and associations between AGD and behavioural parameters have been described (Drickamer et al. 1995; Drickamer 1996; Zehr et al. 2001).
PCBs have been shown to increase as well as decrease male AGD. Treatment of pregnant CD-1 mice with Aroclor 1016 at a dose of 0.1 mg/kg per day increased AGD and prostate size while decreasing epididymal weight in male offspring. This regimen also permanently increased the androgen receptor (AR) binding activity of the prostate. The findings on prostate growth and AR binding were reproduced in vitro (Gupta 2000). In another study, coplanar PCB congeners 77 and 126 given at single doses of 0.1 mg/kg and 0.01 mg/kg, respectively, to pregnant rats on day 15 of pregnancy reduced AGD in male pups and decreased circulating testosterone concentrations in adult male offspring (Faqi et al. 1998). Androgenic effects of PCB treatment on female offspring have been found after intraperitoneal exposure of the dams to PCB 77 or PCB 47 from day 7 to 18 of pregnancy. This treatment increased female AGD, but had no effects on male AGD. Dose levels that affected AGD in females also impaired female sexual receptivity in adult offspring (Wang et al. 2002).
Commercial mixtures of PCBs (Aroclor 1260, Aroclor 1242, Aroclor 1254, Aroclor 1248) as well as some individual congeners (PCB 31, PCB 42) have been shown to interfere with AR-mediated transcription in an in vitro reporter assay system. In addition to an antagonistic action on dihydrotestosterone-elicited transcription, Aroclor 1254 by itself was a weak AR agonist. Androgen ligand binding to the AR was reduced in the presence of PCBs 42, 128 and 138 (Portigal et al. 2002).
Effects on serum steroid concentrations
Concentrations of sexual hormones have been measured by several authors in rats after treatment with PCBs. A1254 or a reconstituted PCB mixture (4 mg/kg per day) decreased E2 and increased testosterone in exposed pregnant female Long-Evans rats. No effects on testosterone were seen in female offspring on PND 21 (testosterone levels were highly variable). Decreased E2 and testosterone in weanling female offspring of female Long-Evans rats exposed until term of pregnancy were found in another study. In adult male offspring both treatments decreased serum testosterone concentrations (Hany et al. 1999b; Kaya et al. 2002). Similar findings on serum testosterone were reported in adult male Wistar rats exposed in utero to PCB 77 or PCB 126 on a single day at the beginning of the fetal period; testosterone levels were highly variable (Faqi et al. 1998). A1254 had no effects on serum and testicular testosterone concentrations in F344 rats after oral treatment with doses up to 25 mg/kg per day from weaning for up to 15 weeks (Gray et al. 1993).
PCB 126 dose-dependently increased E2 in gonadotropin-primed immature female Sprague-Dawley rats while progesterone levels were decreased (Gao et al. 2000).
Vitamin A depletion by PCB exposure
An oral dose of 10 mg/kg per day of A1254 reduces hepatic vitamin A levels in mice and rats (Hallgren et al. 2001) and PCB 77 decreases rat serum levels (Brouwer et al. 1988). One of the reasons may be an increased catabolism due to the PCB-related induction of cytochrome P450 enzymes. In addition, in contrast to T4, which does not interfere with the binding of the retinol-binding protein–retinol complex to transthyretin, the plasma transport of retinol can be disturbed by PCB congeners/hydroxylated metabolites (Brouwer and Van der Berg 1986). In general, such a reduction appeared to be insufficient to induce malformations indicative of vitamin A deficiency. Except for morphological abnormalities of the inner ear in rats and probably in mice, gross morphological changes, mostly cleft palate, have been observed only in mice treated with the planar PCBs 77 and 169 (d’Argy et al. 1987; Marks et al. 1989; Zhao et al. 1997). However, in rat studies embryofetolethality may mask the occurrence of teratogenic effects at doses that induced malformations in mice, and malformed fetuses may have been eliminated by prenatal loss or by maternal cannibalism.
Behavioural effects of PCBs in animal studies
Most of the studies that have been conducted with PCB mixtures or specific congeners suffer from shortcomings. Very few of them comply with current regulatory requirements for reproductive and developmental hazard identification studies. Main problems for the reviewer are listed below.
- –:
-
Group sizes employed in studies in which the mother animals were treated in order to expose the conceptus through the placenta or the newborn through the milk are mostly in the range of less than ten pregnant or lactating females. Such animal numbers are appropriate for a dose-range finding study but not for a main investigation on hitherto unknown outcomes.
- –:
-
Selecting one pup per sex per litter for behavioural studies must be considered insufficient when exposure occurs through the mother animal. This study design assumes that behavioural effects (if present) are 100% penetrant so that each member of a litter is equally well suited to detect the effect. However, it is well known from morphological effect studies that malformations often occur in some of the offspring in a litter while other littermates appear normal. If functional deficiencies follow a similar distribution, effects are likely to be missed with only one litter representative for each sex.
- –:
-
Dose–response or dose–effect relationships cannot be evaluated in many studies because they used only one or two PCB-treated groups.
- –:
-
Plasma and tissue levels of PCBs or their metabolites are frequently not available. However, as the kinetic behaviour of the compounds differs considerably in rodents and man, blood and tissue concentrations must be considered for any risk assessment.
- –:
-
Basic information that must be available for any rational evaluation of a developmental toxicity study, such as adverse effects on the mother, litter sizes, birth weights, postnatal weight gains, and pup mortality, is lacking in many publications on PCBs. Detailed criticism of such studies is included in the supplementary electronic material to this article. Thus, the functional effects in the offspring that are reported cannot be considered in relation to other toxicities.
- –:
-
The studies in rats and mice do not provide individual data. Only in monkey studies are individual performances sometimes reported in the form of a graph. However, studies in non-human primates have even lower group sizes than rodent studies and, for this reason, were not considered in this review. This lack of individual data can make it difficult to evaluate the biological relevance of a finding, especially for behavioural parameters, where the use of mean values may hide the presence of a subgroup of affected animals within a treatment group.
Bearing these difficulties in mind, the reader is directed to a series of diagrams that summarise the effects of individual PCB congeners or commercial and environmentally relevant mixtures on functional development in rodents: Figs. 1, 2 and 3. The emphasis is on single congeners because commercial mixtures have little relevance for current environmental exposure scenarios. Unfortunately, for those persistent congeners (PCBs 138, 180), which together with PCB 153 are most frequently measured in humans, no studies of their effects in animals could be found. Studies have been conducted mainly with different strains of rats with various exposure periods ranging from before mating of the females to the adult age of the offspring. Studies in mice, with a few exceptions, have been performed by only one laboratory where 10-day-old pups were exposed directly by oral gavage (Eriksson 1988; Eriksson and Fredriksson 1996, 1998; Eriksson et al. 1991).
Behavioural aspects that have been studied after PCB exposure fall into four main categories: (1) learning and memory, (2) motor activity, (3) gender dimorphic behaviour, and (4) sexual behaviour. In addition, neurochemical, electrophysiological and thyroid hormone measurements have been performed for several of the compounds in order to elucidate possible mechanisms of behavioural impairment.
Learning and memory
PCBs appear to have detrimental as well as enhancing effects on learning performance. While PCBs 28, 118 and 153 slowed spatial alternation learning in female rats (Schantz et al. 1995; Figs. 1, 4; and Table 2), congeners 77, 95 and 126 had no effects on T-maze performance in either males or females. They increased acquisition of a working memory task in the radial-arm maze spatial learning test, and decreased the error rate in this task (Schantz et al. 1996, 1997b; Figs. 2, 4; and Table 2).
Adverse effects of PCBs on learning in animals and humans have been discussed in the light of the ability of these substances to decrease thyroid hormone plasma levels and the possible decrements in brain function that could arise from hypothyroidism. However, from the data obtained with different PCB congeners, it is unlikely that the learning problems observed in these rat studies are caused by a lack of thyroid hormones. The effects on T-maze performance were similar with PCBs 28, 118 and 153, but only congeners 118 and 153 also affected T4 plasma levels. Plasma concentrations of thyroxine were normal in animals exposed to PCB 28 (Ness et al. 1993).
Another possible mechanism by which learning could be impaired is interference of PCBs and their hydroxylated metabolites with the synthesis of nitric oxide (NO). Endothelial nitric oxide synthase (NOS) in the hippocampus has been implicated in long-term potentiation and learning (references cited in Sharma and Kodavanti 2002). Ortho-substituted PCBs and hydroxylated metabolites, also of non-ortho PCBs, inhibited especially cytosolic NOS (nNOS) in cerebellar, hippocampal and hypothalamic preparations in vitro. The inhibitory concentrations in the assay (10–50 μM) were similar to the concentrations observed in biological fluids and tissues from environmental exposure (Sharma and Kodavanti 2002).
Besides these mechanisms, other neurological systems can be affected by PCBs and may contribute to changes in learning and memory performance: e.g. N-methyl-d-aspartate (NMDA) receptors in the cortex (Altmann et al. 2001). Furthermore, indirect influences via effects on steroids are possible because it is well-known that most transmitter systems, such as serotonin, dopamine, acetylcholine and glutamate, can be influenced by sexual hormones (Auger 2001; Cyr et al. 2001; Haberny et al. 2002; Luconi et al. 2002; Nilsson and Gustafsson 2002; Partridge et al. 2002; Rissman et al. 2002; Rupprecht et al. 2001; Sanchez et al. 2002; Song et al. 2002).
In mice, no improved performance in learning tests was observed with any of the tested PCBs. On the contrary, learning seemed to be impaired. Whereas PCB 118 had no effects on Morris water maze learning in mice exposed on PND10, PCBs 28 and 126 reduced the escape latency after the submerged platform of the Morris water-maze had been moved into a new position, a finding which indicates that treated animals had memorised the original position less consistently than control mice. PCB 77 prolonged latency in an avoidance learning test but at a dose that was overtly toxic to the offspring (Eriksson and Fredriksson 1996, 1998; Tilson et al. 1979; Figs. 3, 4). Impaired performance of passive avoidance after exposure to PCB 77 has been described in rats in the absence of overt toxicity in dams or offspring (Weinand-Härer et al. 1997).
Motor activity
It has been shown that severe reduction of T4 and T3 plasma levels during perinatal and preweaning development by propylthiouracil leads to preweaning decreased or delayed motor activity and to persistent, postweaning hyperactivity in rats (Brosvic et al. 2002). However, in contrast to thyroid hormone levels, motor activity has not been examined as consistently in rat offspring exposed to PCBs (Fig. 4). Data obtained in offspring exposed to PCBs 126 or 153 are consistent with an effect on activity mediated by a decrease of T4 concentration during the first two postnatal weeks (Holene et al. 1998). Adult male offspring exposed to PCB 47 and PCB 77 showed hyperactivity in the open field (Figs. 1, 4). Treatment with PCB 95 during the late embryonal and early fetal period in contrast decreased locomotor activity in an open-field test conducted in adult offspring (Schantz et al. 1997b).
After treatment with PCB 77 from day 7 to 18 of pregnancy, rats showed increased latencies in the haloperidol-induced catalepsy test on postnatal day 100. Similar, but non-significant differences compared to controls, were observed after treatment with PCB 47 or a mixture of these congeners. The endpoint of this test was the time rats required to draw one or both paws from a vertical grid; in modified versions of the test (paws positioned on a bar or block), no differences were detected between PCB-treated rats and controls (Hany et al. 1999a). In another publication by this group, significant effects were reported in another strain of rats, although this time differences were not observed in the “vertical grid test” but in the “bar test” only (Weinand-Härer et al. 1997).
Activity testing in mice exposed on PND10 has produced similar results for four PCB congeners (PCBs 28, 52, 77 and 126). The mice were initially hypoactive compared with controls but displayed an impaired habituation response with time. Therefore, when later time periods of the test are considered, PCB-exposed animals were hyperactive since there was little or no decrease in their ambulation (Eriksson and Fredriksson 1996, 1998; Fig. 3, 4). Changes in activity patterns appeared consistently at the lowest effective doses. Possible mechanisms, however, have not been elucidated.
Gender dimorphic and sexual behaviour
During brain development sexual hormones induce gender-specific differentiation in different brain regions of males and females. Masculinisation or feminisation of the brain leads to different behavioural patterns in adult animals with respect to interactions with possible sexual partners or in behaviour that is unrelated to reproduction. Sweet preference is a sexual dimorphic behaviour that has been used to analyse sex-specific development in the brains of PCB-exposed rats. Females usually display a higher preference for sweetened drinking water than males. Female sweet preference was decreased by PCB 77 (masculinisation of response) whereas male sweet preference was unaffected (Amin et al. 2000). A commercial mixture (Aroclor 1254) was reported to increase female consumption of sweet solutions by approximately 30%, but this was not statistically significant (Hany et al. 1999b). An environmentally relevant mixture increased sweet preference in males at the highest dose tested (Hany et al. 1999b; Kaya et al. 2002; Figs. 2, 4).
Female mating behaviour (lordosis, a measure of receptivity) was found to be decreased with congeners 47 and 77. Both substances also increased anogenital distance in females, which indicates an increased exposure to androgens during the late fetal period (Wang et al. 2002; Figs. 2, 4).
Summary and recommendations
Behavioural effects of PCBs in children have been described in numerous studies. To support the biological plausibility of such effects it has often been argued that corresponding changes have been observed in various animal species; however, many of these animal experiments show methodological flaws. When behavioural parameters are compared with other endpoints for sensitivity across studies, it becomes obvious that only for 4 of the 13 compounds or mixtures with adequate data (PCB 77, 118, 126, and 153) are the behavioural tests at the most sensitive end of the testing battery. For the other cases, changes in enzyme activity, organ weights, postnatal growth or T4 plasma levels can be detected at dose levels below those at which behavioural changes become apparent. As a result of our extensive literature survey we came to the conclusion that, despite the enormous amount of literature, several aspects of PCB-induced developmental toxicity have not been adequately studied so far. For further research in this area the following recommendations may help to close these data gaps:
-
1.
Of those environmentally persistent PCBs that are found preferentially in human tissues and have become standards for PCB exposure (PCBs 138, 153, 180) only PCB 153 has been tested for a number of different endpoints including behaviour. No comparable studies have been found for the other two compounds, which have only been tested as part of mixtures. In order to determine the contribution, if any, of these substances to the adverse effects of PCBs on brain function the individual congeners PCB 138 and PCB 180 should be tested.
-
2.
With respect to environmental exposure, commercial mixtures are not representative. Therefore, it is important that environmentally relevant mixtures are studied to a greater extent. The available data on the reconstituted mixture that was evaluated in this review are incomplete because data on thyroid hormones, metabolic enzymes or behavioural parameters, such as locomotor activity, learning and memory or social/sexual behaviour, are lacking. Additional studies need to be done to characterise the spectrum of effects elicited by mixtures of persistent PCBs.
-
3.
Studies in which exposure is through the dam, either prenatally or postnatally, need to be large enough (group sizes, number of dose groups, number of evaluated offspring per litter) to inspire confidence in the results. In addition to all the parameters normally recorded in regulatory reproductive toxicity studies (maternal body weight gain and clinical signs, implantation sites, live litter size, pup weights at birth and during development, pup mortality data and developmental landmarks), a widespread selection of additional endpoints covering behaviour (learning/memory, activity, sexual and gender-specific), endocrine functions, neurochemistry and measurements of internal exposure should be included. It should be underlined that a general risk exists in studies with small number of animals that the control group is not a valid and representative sample of non-exposed animals, even if the animals are randomly selected. This is especially true for behavioural testing when the variability within and between litters has not been ascertained beforehand.
References
Agrawal AK, Tilson HA, Bondy SC (1981) 3,4,3′,4′-Tetrachlorobiphenyl given to mice prenatally produces long-term decreases in striatal dopamine and receptor binding sites in the caudate nucleus. Toxicol Lett 7:417–424
Allen JR, Barsotti DA (1976) The effects of transplacental and mammary movement of PCBs on infant rhesus monkeys. Toxicology 6:331–340
Altmann L, Weinand-Haerer A, Lilienthal H, Wiegand H (1995) Maternal exposure to polychlorinated biphenyls inhibits long-term potentiation in the visual cortex of adult rats. Neurosci Lett 202:53–56
Altmann L, Lilienthal H, Hany J, Wiegand H (1998) Inhibition of long-term potentiation in developing rat visual cortex but not hippocampus by in utero exposure to polychlorinated biphenyls. Brain Res Dev Brain Res 110:257–260
Altmann L, Mundy WR, Ward TR, Fastabend A, Lilienthal H (2001) Developmental exposure of rats to a reconstituted PCB mixture or Aroclor 1254: Effects on long-term potentiation and [3H]MK-801 binding in occipital cortex and hippocampus. Toxicol Sci 61:321–330
Amin S, Moore RW, Peterson RE, Schantz SL (2000) Gestational and lactational exposure to TCDD or coplanar PCBs alters adult expression of saccharin preference behavior in female rats. Neurotoxicol Teratol 22:675–682
Arnold DL, Bryce F, Stapley R, McGuire PF, Burns D, Tanner JR, Karpinski K (1993a) Toxicological consequences of Aroclor 1254 ingestion by female rhesus (Macaca mulatta) monkeys. Part 1A. Prebreeding phase: clinical health findings. Food Chem Toxicol 31:799–810
Arnold DL, Bryce F, Karpinski K, Mes J, Fernie S, Tryphonas H, Truelove J, McGuire PF, Burns D, Tanner JR, Stapley R, Zawidzka ZZ, Basford D (1993b) Toxicological consequences of Aroclor 1254 ingestion by female rhesus (Macaca mulatta) monkeys. Part 1B. Prebreeding phase: clinical and analytic laboratory findings. Food Chem Toxicol 31:811–824
Arnold DL, Nera EA, Stapley R, Bryce F, Fernie S, Tolnai G, Miller D, Hayward S, Campbell JS, Greer I (1997) Toxicological consequences of Aroclor 1254 ingestion by female rhesus (Macaca mulatta) monkeys and their nursing infants. Part 3: Post-reproduction and pathological findings. Food Chem Toxicol 35:1191–1207
Arnold DL, Bryce F, Mes J, Tryphonas H, Hayward S, Malcolm S (1999) Toxicological consequences of feeding PCB congeners to infant rhesus (Macaca mulatta) and cynomolgus (Macaca fascicularis) monkeys. Food Chem Toxicol 37:153–167
Auger AP (2001) Ligand-independent activation of progestin receptors: relevance for female sexual behaviour. Reproduction 122:847–855
Barsotti DA, Van Miller JP (1984) Accumulation of a commercial polychlorinated biphenyl mixture (Aroclor 1016) in adult rhesus monkeys and their nursing infants. Toxicology 30:31–44
Becker K, Kaus S, Krause C, Lepom P, Schulz C, Seiwert M, Seifert B (2002) German Environmental Survey 1998 (GerES III): environmental pollutants in blood of the German population. Int J Hyg Environ Health 205:297–308
Bonefeld-Jørgensen EC, Andersen HR, Rasmussen TH, Vinggaard AM (2001) Effect of highly bioaccumulated polychlorinated biphenyl congeners on estrogen and androgen receptor activity. Toxicology 158:141–153
Borlak J, Dangers M, Thum T (2002) Aroclor 1254 modulates gene expression of nuclear transcription factors: implications for albumin gene transcription and protein synthesis in rat hepatocyte cultures. Toxicol Appl Pharmacol 181:79–88
Brosvic GM, Taylor JN, Dihoff RE (2002) Influences of early thyroid hormone manipulations: delays in pup motor and exploratory behavior are evident in adult operant performance. Physiol Behav 75:697–715
Brouwer A, Van der Berg KJ (1986) Binding of a metabolite of 3,4,3′,4′-tetrachlorobiphenyl to transthyretin reduces serumvitamin A transport by inhibiting the formation of the protein complex carrying both retinol and thyroxine. Toxicol Appl Pharmacol 85:301–312
Brouwer A, Blaner WS, Kukler A, Van der Berg KJ (1988) Study on the mechanism of interference of 3,4,3′,4′-tetrachlorobiphenyl with the plasma retinol-binding proteins in rodents. Chem Biol Interact 68:203–219
Bushnell PJ, Rice DC (1999) Behavioral assessments of learning and attention in rats exposed perinatally to 3,3′,4,4′,5-pentachlorobiphenyl (PCB 126). Neurotoxicol Teratol 21:381–392
Bushnell PJ, Moser VC, MacPhail RC, Oshiro WM, Derr-Yellin EC, Phillips PM, Kodavanti PRS (2002) Neurobehavioral assessment of rats perinatally exposed to a commercial mixture of polychlorinated biphenyls. Toxicol Sci 68:109–120
Cheek AO, Kow K, Chen J, McLachlan JA (1999) Potential mechanisms of thyroid disruption in humans: Interaction of organochlorine compounds with thyroid receptor transthyretin and thyroid-binding globulin. Environ Health Perspect 107:273–278
Chou SM, Miike T, Payne WM, Davis GJ (1979) Neuropathology of “spinning syndrome” induced by prenatal intoxication with a PCB in mice. Ann N Y Acad Sci 320:373–395
Chung YW, Clemens LG (1999) Effects of perinatal exposure to polychlorinated biphenyls on development of female sexual behavior. Bull Environ Contam Toxicol 62:664–670
Chung YW, Nunez AA, Clemens LG (2001) Effects of neonatal polychlorinated biphenyl exposure on female sexual behavior. Physiol Behav 74:363–370
Collins WT Jr, Capen CC (1980) Fine structural lesions and hormonal alterations in thyroid glands of perinatal rats exposed in utero and by the milk to polychlorinated biphenyls. Am J Pathol 99:25–142
Connor K, Ramamoorthy K, Moore M, Mustain M, Chen I, Safe S, Zacharewski T, Gillesby B, Joyeux A, Balaguer P (1997) Hydroxylated polychlorinated biphenyls (PCBs) as estrogens and antiestrogens: structure–activity relationships. Toxicol Appl Pharmacol 145:111–123
Cooke PS, Zhao YD, Hansen LG (1996) Neonatal polychlorinated biphenyl treatment increases adult testis size and sperm production in rats. Toxicol Appl Pharmacol 136:112–117
Crofton KM, Rice DC (1999) Low-frequency hearing loss following perinatal exposure to 3,3′,4,4′,5-pentachlorobiphenyl (PCB 126) in rats. Neurotoxicol Teratol 21:299–301
Crofton KM, Ding D, Padich R, Taylor M, Henderson D (2000a) Hearing loss following exposure during development to polychlorinated biphenyls: a cochlear site of action. Hear Res 144:196–204
Crofton KM, Kodavanti PRS, Casey AC, Kehn LS (2000b) PCBs, thyroid hormones, and ototoxicity in rats: cross-fostering experiments demonstrate the impact of postnatal lactation exposure. Toxicol Sci 57:131–140
Cyr M, Ghribi O, Thibault C, Morissette M, Landry M, Di Paolo T (2001) Ovarian steroids and selective estrogen receptor modulators activity on rat brain NMDA and AMPA receptors. Brain Res Rev 37:153–161
d’Argy R, Dencker L, Klasson-Wehler E, Bergman A, Darnerud PO, Brandt I (1987) 3,3′,4,4′-Tetrachlorobiphenyl in pregnant mice: embryotoxicity, teratogenicity and toxic effects on the cultured embryonic thymus. Pharmacol Toxicol 61:53–57
Drickamer LC (1996) Intra-uterine position and anogenital distance in house mice: consequences under field conditions. Anim Behav 51:925–934
Drickamer LC, vom Saal FS, Marriner LM, Mossman CA (1995) Anogenital distance and dominance status in male house mice (Mus domesticus). Aggress Behav 21:301–309
Eriksson P (1988) Effects of 3,3′,4,4′-tetrachlorobiphenyl in the brain of the neonatal mouse. Toxicology 49:43–48
Eriksson P, Fredriksson A (1996) Developmental neurotoxicity of four ortho-substituted polychlorinated biphenyls in the neonatal mouse. Environ Toxicol Pharmacol 1:155–165
Eriksson P, Fredriksson A (1998) Neurotoxic effects in adult mice neonatally exposed to 3,3′,4,4′,5-pentachlorobiphenyl or 2,3,3′,4,4′- pentachlorobiphenyl: changes in brain nicotinic receptors and behaviour. Environ Toxicol Pharmacol 5:17–27
Eriksson P, Lundkvist U, Fredriksson A (1991) Neonatal exposure to spontaneous behaviour and cholinergic muscarinic receptors in the adult mouse. Toxicology 69:27–34
Esser C, Lai Z, Gleichmann E (1994) Proliferation inhibition and CD4/CD8 thymocyte subset skewing by in vivo exposure of C57BL/6 mice to Ah receptor-binding 3,3′,4,4′-tetrachlorobiphenyl. Exp Clin Immunogenet 11:75–85
Faqi AS, Dalsenter PR, Merker H-J, Chahoud I (1998) Effects on developmental landmarks and reproductive capability of 3,3′,4,4′-tetrachlorobiphenyl and 3,3′,4,4′,5-pentachlorobiphenyl in offspring of rats exposed during pregnancy. Hum Exp Toxicol 17:365–372
Gao X, Terranova PF, Rozman KK (2000) Effects of polychlorinated dibenzofurans biphenyls and their mixture with dibenzo-p-dioxins on ovulation in the gonadotropin-primed immature rat: support for the toxic equivalency concept. Toxicol Appl Pharmacol 163:115 −124
Geller AM, Bushnell PJ, Rice DC (2000) Behavioral and electrophysiological estimates of visual thresholds in awake rats treated with 3,3′,4,4′,5-pentachlorobiphenyl (PCB 126). Neurotoxicol Teratol 22:521–531
Geller AM, Oshiro WM, Haykal-Coates N, Kodavanti PRS, Bushnell PJ (2001) Gender-dependent behavioral and sensory effects of a commercial mixture of polychlorinated biphenyls (Aroclor 1254) in rats. Toxicol Sci 59:268–277
Gilbert ME, Crofton KM (1999) Developmental exposure to a commercial PCB mixture (Aroclor 1254) produces a persistent impairment in long-term potentiation in the rat dentate gyrus in vivo. Brain Res 850:87–95
Gilbert ME, Mundy WR, Crofton KM (2000) Spatial learning and long-term potentiation in the dentate gyrus of the hippocampus in animals developmentally exposed to Aroclor 1254. Toxicol Sci 57:102 −111
Goldey ES, Crofton KM (1998) Thyroxine replacement attenuates hypothyroxinemia hearing loss and motor deficits following developmental exposure to Aroclor 1254 in rats. Toxicol Sci 45:94–105
Goldey ES, Kehn LS, Lau C, Rehnberg GL, Crofton KM (1995a) Developmental exposure to polychlorinated biphenyls (Aroclor 1254) reduces circulating thyroid hormone concentrations and causes hearing deficits in rats. Toxicol Appl Pharmacol 135:77–88
Goldey ES, Kehn LS, Rehnberg GL, Crofton KM (1995b) Effects of developmental hypothyroidism on auditory and motor function in the rat. Toxicol Appl Pharmacol 135:67–76
Gray LE Jr, Ostby J, Marshall R, Andrews J (1993) Reproductive and thyroid effects of low-level polychlorinated biphenyl (Aroclor 1254) exposure. Fundam Appl Toxicol 20/3:288–294
Gray LE Jr, Wolf C, Lambright C, Mann P, Price M, Cooper RL, Ostby J (1999) Administration of potentially antiandrogenic pesticides (procymidone linuron iprodione chlozolinate pp′-DDE and ketoconazole) and toxic substances (dibutyl- and diethylhexyl phthalate PCB 169 and ethane dimethane sulphonate) during sexual differentiation produces diverse profiles of reproductive malformations in the male rat. Toxicol Ind Health 15:94–118
Gupta C (2000) Reproductive malformation of the male offspring following maternal exposure to estrogenic chemicals. Proc Soc Exp Biol Med 224:61–68
Haberny KA, Paule MG, Scallet AC, Sistare FD, Lester DS, Hanig JP, Slikker W (2002) Ontogeny of the N-methyl-d-aspartate (NMDA) receptor system and susceptibility to neurotoxicity. Toxicol Sci 68:9–17
Hall JM, McDonnell DP, Korach KS (2002) Allosteric regulation of estrogen receptor structure function and coactivator recruitment by different estrogen response elements. Mol Endocrinol 16:469–486
Hallgren S, Sinjari T, Hakansson H, Darnerud PO (2001) Effects of polybrominated diphenyl ethers (PBDEs) and polychlorinated biphenyls (PCBs) on thyroid hormone and vitamin A levels in rats and mice. Arch Toxicol 75:200–208
Hany J, Lilienthal H, Roth-Härer A, Ostendorp G, Heinzow B, Winneke G (1999a) Behavioral effects following single and combined maternal exposure to PCB 77 (3,4,3′,4′-tetrachlorobiphenyl) and PCB 47 (2,4,2′,4′-tetrachlorobiphenyl) in rats. Neurotoxicol Teratol 21:147–156
Hany J, Lilienthal H, Sarasin A, Roth-Härer A, Fastabend A, Dunemann L, Lichtensteiger W, Winneke G (1999b) Developmental exposure of rats to a reconstituted PCB mixture or Aroclor 1254: effects on organ weights aromatase activity sex hormone levels and sweet preference behavior. Toxicol Appl Pharmacol 158:231–243
Hernandez-Tristan R, Arevalo C, Canals S (1999) Effect of prenatal uterine position on male and female rats sexual behavior. Physiol Behav 67:401–408
Herr DW, Graff JE, Derr-Yellin EC, Crofton KM, Kodavanti PRS (2001) Flash- somatosensory- and peripheral nerve-evoked potentials in rats perinatally exposed to Aroclor 1254. Neurotox Teratol 23:591–601
Holene E, Nafstad I, Skaare J U, Sagvolden T (1998) Behavioural hyperactivity in rats following postnatal exposure to sub-toxic doses of polychlorinated biphenyl congeners 153 and 126. Behav Brain Res 94:213–224
Ikeda M, Inukai N, Mitsui T, Sone H, Yonemoto J, Tohyama C, Tomita T (2002) Changes in fetal brain aromatase activity following in utero 2,3,7,8-tetrachlorodibenzo-p-dioxin exposure in rats. Environ Toxicol Pharmacol 11:1–7
Kato Y, Haraguchi K; Shibahara T, Yumoto S, Masuda Y, Kimura R (1999) Reduction of thyroid hormone levels by methylsulfonyl metabolites of tetra- and pentachlorinated biphenyls in male Sprague-Dawley rats. Toxicol Sci 48:51–54
Kaya H, Hany J, Fastabend A, Roth-Härer A, Winneke G, Lilienthal H (2002) Effects of maternal exposure to a reconstituted mixture of polychlorinated biphenyl on sex-dependent behaviors and steroid hormone concentrations in rats: dose–response relationship. Toxicol Appl Pharmacol 178:71–81
Kester MHA, Bulduk S, Tibboel D, Meinl W, Glatt H, Falany CN, Coughtrie MHW, Bergman A, Safe SH, Kuiper GGJM, Schuur AG, Brouwer A, Visser TJ (2000) Potent inhibition of estrogen sulfotransferase by hydroxylated PCB metabolites: a novel pathway explaining the estrogenic activity of PCBs. Endocrinology 141:1897–1900
Khan MA, Lichtensteiger CA, Faroon O, Mumtaz M, Schaeffer DJ, Hansen LG (2002) The hypothalamo–pituitary–thyroid (HPT) axis: a target of nonpersistent ortho-substituted PCB congeners. Toxicol Sci 65:52–61
Kimbrough RD, Doemland M, Krouskas CA (2001) Analysis of research studying the effects of polychlorinated biphenyls and related chemicals on the neurobehavioral development of children. Vet Human Toxicol 43:220–228
Kramer VJ, Helferich WG, Bergman A, Klasson-Wehler E, Giesy JP (1997) Hydroxylated polychlorinated biphenyl metabolites are anti-estrogenic in a stably transfected human breast adenocarcinoma (MCF7) cell line. Toxicol Appl Pharmacol 144:363–376
Kuiper GGJM, Lemmen JG, Carlsson B, Corton JC, Safe SH, van der Saag PT, van der Burg P, Gustafsson JA (1998) Interaction of estrogenic chemicals and phytoestrogens with estrogen receptor β. Endocrinology 139:4252–4263
Lackmann GM (2002) Polychlorinated biphenyls and hexachlorobenzene in full-term neonates—reference values updated. Biol Neonate 81:82–85
Lans MC, Spiertz C, Brouwer A, Koeman JH (1994) Different competition of thyroxine binding to transthyretin and thyroxine-binding globulin by hydroxy-PCBs PCDDs and PCDFs. Eur J Pharmacol 270:129–136
Layton AC, Sanseverino J, Gregory BW, Easter JP, Sayler GS, Schultz TW (2002) In vitro estrogen receptor binding of PCBs: Measured activity and detection of hydroxylated metabolites in a recombinant yeast assay. Toxicol Appl Pharmacol 180:157–163
Levin ED, Schantz L, Bowman RE (1988) Delayed spatial alternation deficits resulting from perinatal PCB exposure monkeys. Arch Toxicol 62:267–273
Li MH, Hansen LG, (1995) Uterotropic and enzyme induction effects of 2,2′,5-trichlorobiphenyl. Bull Environ Contam Toxicol 54:494–500
Lilienthal H, Winneke G (1991) Sensitive periods for behavioral toxicity of polychlorinated biphenyls: determination by cross-fostering in rats. Fundam Appl Toxicol 17:368–375
Lilienthal H, Neuf M, Munoz C, Winneke G (1990) Behavioral effects of pre- and postnatal exposure to a mixture of low chlorinated PCBs in rats. Fundam Appl Toxicol 15:457–467
Longnecker MP, Gladen BC, Patterson DG, Rogan WJ (2000) Polychlorinated biphenyl (PCB) exposure in relation to thyroid hormone levels in neonates. Epidemiology 11:249–254
Luconi M, Forti G, Baldi E (2002) Genomic and nongenomic effects of estrogens: molecular mechanisms of action and clinical implications for male reproduction. J Steroid Biochem Mol Biol 80:369–381
Marks TA, Kimmel GL, Staples RE (1981) Influence of symmetrical polychlorinated biphenyl isomers on embryo and fetal development in mice. I. Teratogenicity of 3,3′,4,4′,5,5′-hexachlorobiphenyl. Toxicol Appl Pharmacol 61:269–276
Marks TA, Kimmel GL, Staples RE (1989) Influence of symmetrical polychlorinated biphenyl isomers on embryo and fetal development in mice. II. Comparison of 4,4′-dichlorobiphenyl, 3,3′,4,4′-tetrabiphenyl, 3,3′,5,5′-tetrachlorobiphenyl and 3,3′,4,4′-tetramethylbiphenyl. Fundam Appl Toxicol 13:681–693
Meerts IA, Assink Y, Cenijn PH, Van Den Berg JH, Weijers BM, Bergman A, Koeman JH, Brouwer A (2002) Placental transfer of a hydroxylated polychlorinated biphenyl and effects on fetal and maternal thyroid hormone homeostasis in the rat. Toxicol Sci 68:361–371
Mele PC, Bowman RE, Levin ED (1986) Behavioral evaluation of perinatal PCB exposure rhesus monkeys: Fixed-interval performance and reinforcement omission. Neurobehav Toxicol Teratol 8:131–138
Mes J, Arnold DL, Bryce F (1994) Determination of polychlorinated biphenyls postpartum blood, adipose tissue, and milk from female rhesus monkeys and their offspring after prolonged dosing with aroclor 1254. J Anal Toxicol 18:29–35
Miyakawa T, Yared E, Pak JH, Huang FL, Huang KP, Crawley JN (2001) Neurogranin null mutant mice display performance deficits on spatial learning tasks with anxiety related components. Hippocampus 11:763–775
Morse DC, Groen D, Veerman M, van Amerongen CJ, Koeter HBWM, Smits van Prooije AE, Visser TJ, Brouwer A (1993) Interference of polyclorinated biphenyls in hepatic and brain thyroid hormone metabolism in fetal and neonatal rats. Toxicol Appl Pharmacol 122:27–33
Morse DC, Wehler EK, van de Pas M, de Bie AT, van Bladeren P, Brouwer A (1995) Metabolism and biochemical effects of 3,3′,4,4′-tetrachlorobiphenyl in pregnant and fetal rats. Chem Biol Interact 95:41–56
Morse DC, Wehler EK, Wesseling W, Koeman JH, Brouwer A (1996) Alterations in rat brain thyroid hormone status following pre- and postnatal exposure to polychlorinated biphenyls (Aroclor 1254). Toxicol Appl Pharmacol 136:269–279
Nawrot TS, Staessen JA, Den Hond EM, Koppen G, Schoeters G, Fagard R, Thijs L, Winneke G, Roels HA (2002) Host and environmental determinants of polychlorinated aromatic hydrocarbons in serum of adolescents. Environ Health Perspect 110:583–589
Ness DK, Schantz SL, Moshtaghian J, Hansen LG (1993) Effects of perinatal exposure to specific PCB congeners on thyroid hormone concentrations and thyroid histology in the rat. Toxicol Lett 68:311–323
Nilsson S, Gustafsson JA (2002) Biological role of estrogen and estrogen receptors. Crit Rev Biochem Mol Biol 37:1–28
Partridge JG, Apparsundaram S, Gerhardt GA, Ronesi J, Lovinger DM (2002) Nicotinic acetylcholine receptors interact with dopamine in induction of striatal long-term depression. J Neurosci 22:2541–2549
Persky V, Turyk M, Anderson HA, Hanrahan LP, Falk C, Steenport DN, Chatterton R, Freels S (2001) The effects of PCB exposure and fish consumption on endogenous hormones. Environ Health Perspect 109:1275–1283
Portigal CL, Cowell SP, Fedoruk MN, Butler CM, Rennie PS, Nelson CC (2002) Polychlorinated biphenyls interfere with androgen-induced transcriptional activation and hormone binding. Toxicol Appl Pharmacol 179:185–194
Ribas-Fito N, Sala M, Kogevinas M, Sunyer J (2001) Polychlorinated biphenyls (PCBs) and neurological development in children: a systematic review. J Epidemiol Community Health 55:537–546
Rice DC (1999a) Effect of exposure to 3,3′,4,4′,5-pentachlorobiphenyl (PCB 126) throughout gestation and lactation on development and spatial delayed alternation performance in rats. Neurotoxicol Teratol 21:59–69
Rice DC (1999b) Behavioral impairment produced by low-level postnatal PCB exposure monkeys. Environ Res 80:S113–S121
Rice DC, Hayward S (1997) Effects of postnatal exposure to a PCB mixture in monkeys on nonspacial discrimination reversal and delayed alternation performance. Neurotoxicology 18:479–494
Rissman EF, Heck AL, Leonard JE, Shupnik MA, Gustafsson JA (2002) Disruption of estrogen receptor β gene impairs spatial learning in female mice. Proc Natl Acad Sci USA 99:3996–4001
Robbins J (1996) Thyroid hormone transport proteins and the physiology of hormone binding. In: Braverman LE, Utiger RD (eds) Werner and Ingbar’s The thyroid: a fundamental and clinical text. Lippincott, Philadelphia, pp 96–110
Roegge CS, Seo BW, Crofton KM, Schantz SL (2000) Gestational–lactational exposure to Aroclor 1254 impairs radial-arm maze performance in male rats. Toxicol Sci 57:121–130
Roth-Härer A, Lilienthal H, Bubser M, Kronthaler U, Mundy R, Ward R, Schmidt W, Winterhoff H, Winneke G (2001) Neurotransmitter concentrations and binding at dopamine receptors in rats after maternal exposure to 3,4,3′,4′-tetrachlorobiphenyl: the role of reduced thyroid hormone concentrations. Environ Toxicol Pharmacol 9:103–115
Rupprecht R, di Michele F, Hermann B, Ströhle A, Lancel M, Romeo E, Holsboer F (2001) Neuroactive steroids: molecular mechanisms of action and implications for neuropsychopharmacology. Brain Res Rev 37:59–67
Safe S, Wang F, Porter W, Duan R, McDougal A (1998) Ah receptor agonists as endocrine disruptors: antiestrogenic activity and mechanisms. Toxicol Lett 103:343–347
Sager DB (1983) Effect of postnatal exposure to polychlorinated biphenyls on adult male reproductive function. Environ Res 31:76–94
Sager DB, Girard DM (1994) Long-term effects on reproductive parameters in female rats after translactational exposure to PCBs. Environ Res 66:52–76
Sager DB, Shih-Schroeder W, Girard D (1987) Effects of early postnatal exposure to polychlorinated biphenyls (PCBs) on fertility in male rats. Bull Environ Contam Toxicol 38:946–953
Sager DB, Girard D, Nelson D (1991) Early postnatal exposure to PCBs: sperm function in rats. Environ Toxicol Chem 10:737–746
Sanchez R, Nguyen D, Rocha W, White J H, Mader S (2002) Diversity in the mechanisms of gene regulation by estrogen receptors. Bioessays 24:244–254
Sauer PJ, Huisman M, Koopman-Esseboom C, Morse DC, Smits-van Prooije AE, van de Berg KJ, Tuinstra LG, Van der Paauw CG, Boersma ER, Weisglas-Kuperus N (1994) Effects of polychlorinated biphenyls (PCBs) and dioxins on growth and development. Hum Exp Toxicol 13:900–906
Savu L, Vranckx R, Maya M, Gripois D, Blouquit MF, Nunez EA (1989) Thyroxine-binding globulin and thyroxine-binding prealbumin in hypothyroid and hyperthyroid developing rats. Biochim Biophys Acta 992:379–384
Schantz SL, Levin ED, Bowman RE, Heironimus MP, Laughlin NK (1989) Effects of perinatal PCB exposure on discrimination-reversal learning monkeys. Neurotoxicol Teratol 11:243–250
Schantz SL, Levin ED, Bowman RE (1991) Long-term neurobehavioral effects of perinatal polychlorinated biphenyl (PCB) exposure in monkeys. Environ Toxicol Chem 10:747–756
Schantz SL, Moshtaghian J, Ness DK (1995) Spatial learning deficits in adult rats exposed to ortho-substituted PCB congeners during gestation and lactation. Fundam Appl Toxicol 26:117–126
Schantz SL, Seo BW, Moshtaghian J, Peterson RE, Moore RW (1996) Effects of gestational and lactational exposure to TCDD or coplanar PCBs on spatial learning. Neurotoxicol Teratol 18:305–313
Schantz SL, Seo BW, Moshtaghian J, Amin S (1997a) Developmental exposure to polychlorinated biphenyls or dioxin: Do changes in thyroid function mediate effects on spatial learning? Am Zool 37:399–408
Schantz SL, Seo BW, Wong PW, Pessah IN (1997b) Long-term effects of developmental exposure to 2,2′,3,5′,6-pentachlorobiphenyl (PCB 95) on locomotor activity spatial learning and memory and brain ryanodine binding. Neurotoxicology 18:457–467
Schantz SL, Widholm JJ, Rice DC (2003) Effects of PCB exposure on neuropsychological function in children. Environ Health Perspect 111:357–576
Schuur AG, Legger FF, van Meeteren ME, Moonen MJH, van Leeuwen-Bol I, Bergman A, Visser TJ, Brouwer A (1998a) In vitro inhibition of thyroid hormone sulfation by hydroxylated metabolites of halogenated aromatic hydrocarbons. Chem Res Toxicol 11:1075–1081
Schuur AG, Brouwer A, Bergman A, Coughtrie MWH, Visser TJ (1998b) Inhibition of thyroid hormone sulfation by hydroxylated metabolites of polychlorinated biphenyls. Chem Biol Interact 109:293–297
Seegal RF, Brosch KO, Okoniewski RJ (1997) Effects of in utero and lactational exposure of the laboratory rat to 2,4,2′,4′- and 3,4,3′,4′-tetrachlorobiphenyl on dopamine function. Toxicol Appl Pharmacol 146:95–103
Seo BW, Li MH, Hansen LG, Moore RW, Peterson RE, Schantz SL (1995) Effects of gestational and lactational exposure to coplanar polychlorinated biphenyl (PCB) congeners or 2,3,7,8-tetrachlorodibenzo-p-dioxin (TCDD) on thyroid hormone concentrations in weanling rats. Toxicol Lett 78:253–262
Sharma R, Kodavanti PRS (2002) In vitro effects of polychlorinated biphenyls and hydroxy metabolites on nitric oxide synthases in rat brain. Toxicol Appl Pharmacol 178:127–136
Simmons DL, Valentine DM, Bradshaw WS (1984) Different patterns of developmental toxicity in the rat following prenatal administration of structurally diverse chemicals. J Toxicol Environ Health 14:121–136
Song RXD, McPherson RA, Adam L, Bao YD, Shupnik M, Kumar R, Santen RJ (2002) Linkage of rapid estrogen action to MAPK activation by ERα–Shc association and Shc pathway activation. Mol Endocrinol 16:116–127
Tilson HA, Davis G, McLachlan JA, Lucier GW (1979) The effects of polychlorinated biphenyls given prenatally on the neurobehavioral development of mice. Environ Res 18:466–474
Tryphonas H, Luster MI, Schiffman G, Dawson L-L, Hodgen M, Germolec D, Hayward S, Bryce F, Loo JCK, Mandy F, Arnold DL (1991) Effect of chronic exposure of PCB (Aroclor 1254) on specific and nonspecific immune parameters the rhesus (Macaca mulatta) monkey. Fundam Appl Toxicol 16:773–786
Umweltbundesamt (Kommission Human-Biomonitoring) (1999) Stoffmonographie PCB—Referenzwerte für Blut. Bundesgesundhbl 42:511–521
Umweltbundesamt (Kommission Human-Biomonitoring) (2002) Available as online publication: http://www.umweltbundesamt.de/uba-info-daten/daten/monitor/hbm-tabellen.htm#Tabelle 2
Vandenbergh JG, Huggett CL (1995) The anogenital distance index a predictor of the intrauterine position effects on reproduction in female house mice. Lab Animal Sci 45 567–573
Vansell NR, Klaassen CD (2002) Effect of microsomal enzyme inducers on the biliary excretion of triiodothyronine (T3) and its metabolites. Toxicol Sci 65:14–191
Voorspoels S, Covaci A, Maervoet J, Schepens P (2002) Relationship between age and levels of organochlorine contaminants in human serum of a Belgian population. Bull Environ Contam Toxicol 69:22–29
Vranckx R, Savu L, Maya M, Nunez EA (1990) Characterization of a major development-regulated serum thyroxine-binding globulin in the euthyroid mouse. Biochem J 271:373–379
Wang XQ, Fang J, Nunez AA, Clemens LG (2002) Developmental exposure to polychlorinated biphenyls affects sexual behavior of rats. Physiol Behav 75:689–696
Weinand-Härer A, Lilienthal H, Bucholski K-A, Winneke G (1997) Behavioral effects of maternal exposure to an ortho-chlorinated or a coplanar PCB congener in rats. Environm Toxicol Pharmacol; 3:97–103
White JT, Kelly JW (2001) Support for the multigenic hypothesis of amyloidosis: The binding stoichiometry of retinol-binding protein vitamin A and thyroid hormone influences transthyretin amyloidogenicity in vitro. Proc Natl Acad Sci USA 98:13019–13024
Zehr JL, Gans SE, McClintock MK (2001) Variation in reproductive traits is associated with short anogenital distance in female rats. Dev Psychobiol 38:229–238
Zhao F, Mayura K, Harper N, Safe SH, Phillips TD (1997) 3,3′,4,4′,5-pentachlorobiphenyl-induced fetal cleft palate and immunotoxicity in C57BL/6 mice by 2,2′,4,4′,5,5′-hexachlorobiphenyl. Chemosphere 34:1605–1613
Zoeller RT, Dowling AL, Vas AA (2000) Developmental exposure to polychlorinated biphenyls exerts thyroid hormone-like effects on the expression of RC3/neurogranin and myelin basic protein messenger ribonucleic acids in the developing rat brain. Endocrinology 141:181 −189
Acknowledgements
The financial support of EURO CHLOR, Brussels, is gratefully acknowledged. The invaluable assistance of Mrs. Barbara Steyn and Mrs. Heidi Pretorius in the preparation of this manuscript is greatly appreciated. We thank Harald Weinrich for the adaptation of the graphs for the internet.
Author information
Authors and Affiliations
Corresponding author
Additional information
An erratum to this article can be found at http://dx.doi.org/10.1007/s00204-004-0583-y
Rights and permissions
About this article
Cite this article
Ulbrich, B., Stahlmann, R. Developmental toxicity of polychlorinated biphenyls (PCBs): a systematic review of experimental data. Arch Toxicol 78, 252–268 (2004). https://doi.org/10.1007/s00204-003-0519-y
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00204-003-0519-y