Abstract
Bisphenol A (BPA) is a monomer used mainly in the synthesis of polycarbonates and epoxy resins. Percutaneous absorption is the second source of exposure, after inhalation, in the work environment. However, studies on this route of absorption are lacking or incomplete. In this study, percutaneous BPA absorption was measured in vivo and ex vivo in the rat, and ex vivo in humans. An approximately 12-fold difference in permeability between rat skin and human skin was found, with permeability being higher in the rat. In addition, inter- and intra-individual variability of up to tenfold was observed in humans. No accumulation of BPA in the skin was found during exposure. The skin clearance rate following exposure was estimated at 0.4 μg/cm²/h. Ex vivo and in vivo percutaneous absorption fluxes of BPA in the rat were in the same range (about 2.0 μg/cm²/h), suggesting that extrapolation to the in vivo situation in humans may be possible. The European tolerable daily intake (TDI) of BPA is 50 μg/kg body weight. However, many research projects have highlighted the significant effects of BPA in rodents at doses lower than 10 μg/kg/day. A 1-h occupational exposure over 2,000 cm² (forearms and hands) may lead to a BPA absorption of 4 μg/kg/day. This is 8% of the European TDI and is very close to the value at which effects have been observed in animals. This absorption must therefore be taken into account when evaluating risks of BPA exposure, at least until more relevant results on the toxicity of BPA in humans are available.
Similar content being viewed by others
Introduction
Bisphenol A (BPA, CAS No. 80-05-7) is a plastic monomer and plasticizer, primarily used in the production of polycarbonate plastics and epoxy resins. It is one of the world’s highest production-volume chemicals, with more than 2 million metric tons produced worldwide in 2003. A 6–10% yearly growth in demand for BPA is predicted (Burridge 2003). In its assessment of the risks associated with BPA, published in January 2007, the European Food Safety Authority (EFSA) set a full tolerable daily intake (TDI) of 50 μg/kg body weight/day. In March 2010, EFSA received a request from the European Commission to take into account in its risk assessment any other new scientific evidence that may be available and to review scientific arguments supplied by Denmark in support of the government’s decision to ban the use of BPA in food contact materials for infants aged from 0 to 3 years. EFSA updated its advice on BPA in September 2010. Only in vivo studies complying with certain inclusion criteria were considered for the purpose of this risk assessment in order to assess the validity and/or applicability of the individual findings to human risk assessment. They concluded that following a detailed and comprehensive review of recent scientific literature on the toxicity of BPA at low doses, they could not identify any new evidence that would lead them to revise the current TDI for BPA of 50 μg/kg body weight set in its 2006 opinion and re-confirmed in its 2008 opinion.
Following oral dosing in humans, BPA was rapidly conjugated and excreted in urine due to the absence of enterohepatic circulation (Völkel et al. 2002). In the same way, BPA given orally or intravenously to cynomolgus monkeys was also mainly excreted in urine (Kurebayashi et al. 2002). In contrast, after oral or intravenous administration in rats, the major excretion route was feces (Kurebayashi et al. 2003; Domoradzki et al. 2003), suggesting that BPA is mainly metabolized to BPA-glucuronide and excreted into feces through the bile and subject to enterohepatic circulation in rats, irrespective of dose and administration route. In December 2004, vom Saal and Hughes (2005) reviewed 115 published studies that used low doses of BPA, below the previous lowest observed adverse effect level (LOAEL) of 50 μg/kg/day. In vivo estrogenic activity of BPA was reported in 94 of the studies reviewed, and 31 reported effects caused by doses of BPA at or below the reference dose of 50 μg/kg/day. Many of the adverse effects observed (sexual maturation, hormone levels in blood, fertility, immune function, enzyme activity, brain chemistry, behavior, etc.) were due to exposure during early development (gestation and/or lactation), but effects due to exposure postweaning, right up to adulthood, have been reported (Al-Hiyasat et al. 2002; Palanza et al. 2002; Rubin et al. 2001). Sekizawa (2008) reported that adverse effects on development, reproduction, morphology, behavior, and sexual dimorphism were observed in rats and mice at doses below 10 μg/kg/day. Based on human samples, Völkel et al. (2008) determined an average urinary concentration of 2.5 μg/l and used this value for risk assessment. The authors then determined total BPA in 147 urine samples and found concentrations between <LOD (0.3 μg/l) and 9.3 μg/l. In another study carried out in the United States, Calafat et al. (2005) measured urinary BPA in a reference population of 394 adults. BPA concentrations ≥ 0.1 μg/l were detected in 95% of the samples examined, with geometric mean and median concentrations of 1.33 and 1.28 μg/l, respectively. In a more recent study, high urinary BPA concentrations were associated with cardiovascular diagnoses, diabetes, and clinically abnormal concentrations of the liver enzymes γ-glutamyltransferase and alkaline phosphatase (Lang et al. 2008). Thus, most humans are exposed to BPA at sufficient levels for it to be detectable in the urine and high urinary concentrations have been linked to various diseases.
In the United States, the National Toxicology Program (NTP) Center for the Evaluation of Risks to Human Reproduction (CERHR) evaluated the potential for BPA to cause adverse effects on reproduction and development in humans (NTP-CEHRH 2008). The NTP concluded that there were some concerns about the effects on the brain, behavior, and prostate gland in fetuses, infants, and children, at current levels of human exposure to BPA. However, they also concluded that current levels of human exposure to bisphenol A in fetuses, infants, and children were of minimal concern for effects on the mammary gland and an earlier age for puberty in females. In addition, they expressed minimal concern that exposure to BPA at higher levels in occupational settings would cause reproductive effects in workers. At the end of 2008, the National Institute of Environmental Health Sciences (NIEHS) and NTP published a request for information (RFI) on their website, seeking input on a number of key research areas identified in recent evaluations of BPA. This should help focus future research and testing of BPA.
It is commonly assumed that human exposure to chemical toxicants occurs mainly through breathing, but toxic materials used in the workplace can often result in absorption through the skin. Despite the growing interest of regulatory authorities for BPA effects on organisms, data on percutaneous absorption of BPA are almost impossible to obtain. To our knowledge, there is only one study that quantifies the transcutaneous penetration of BPA in pig skin (Kaddar et al. 2008). Unfortunately, the results of Kaddar et al.’s study do not allow an absorption flux for BPA to be calculated.
The aim of the present study was to determine the percutaneous absorption flux for BPA. First of all, using complementary techniques, the absorption flux was determined in vivo and ex vivo in male Sprague–Dawley rats. Then, human skin samples were exposed ex vivo to BPA in the same conditions as those used for rats. Results from the two species were then compared with a view to extrapolating from the ex vivo fluxes to reflect what happens in human skin in situ.
Materials and methods
Test and control substances
Radiolabeled bisphenol A ([ring 14C(U)]-bisphenol A) was supplied by Moravek Biochemicals, Inc. (California, USA) with a specific radioactivity of 7.4 GBq/mmol (200 mCi/mmol). Radiochemical purity, as determined by the supplier, was 99.7% by HPLC. The radiolabeled compound was stored at −20°C. The working solutions were prepared 1 day before their administration, by weighing [14C]-BPA, unlabeled BPA (Sigma–Aldrich Chimie Sarl, Saint Quentin Fallavier, France) and vehicle with a precision balance, verified as part of a quality assurance process. A radiochemical purity control was performed on each working solution by HPLC after dilution of the stock solution. Liquid scintillation (Tri-Carb 2900TR, Perkin Elmer, the Netherlands) was used to determine radiochemical concentration on triplicate samples. Unlabeled BPA chemical purity, as indicated by the supplier, was more than 99%. The unlabeled compound was stored in an amber bottle at room temperature. All other chemicals and materials were from Sigma–Aldrich, unless specified, and were of the highest available purity.
Animals
Sprague–Dawley male rats (Charles River, Saint Germain sur L’Arbresle, France) weighing 250–300 g were used for the in vivo studies. The animals were kept at constant temperature and humidity with free access to a commercial rodent diet (food pellets, Scientific Animal Food and Engineering, Augy, France). Animals were acclimatized to the laboratory conditions for at least 4 days before the start of experiments.
Mass balance after intravenous administration of [14C]-BPA
[14C]-BPA was administered intravenously in a 10% Cremophor EL® solution (1 ml/kg) into the dorsal vein of the penis of lightly isoflurane anaesthetized rats. The doses tested were 10, 50, 100, 500, and 1,000 μg/kg of BPA (n = 3–6). Precise individual doses were determined by weighing the syringe before and after each injection. The radiocarbon dose was about 0.7 MBq/kg (20 μCi/kg). After injection, the animals were immediately placed in individual metabolism cages for urine and feces collection. Urine was collected at 4, 8, 24, 30, 48, and 72 h. Feces were collected every 24 h. At the end of the experiment, the animals were euthanized by a lethal intravenous injection of sodium pentobarbital (CEVA SANTE ANIMALE, France), under light isoflurane anesthesia. The remaining radioactivity was determined after digesting carcasses in an alcohol/KOH mixture (1.5 M, H2O/ethanol, V/V, 1/4).
In vivo percutaneous penetration and absorption of BPA
One day before administration, hair was clipped in the shoulder and back region. On the day of administration the skin surface was gently wiped with acetone to remove sebum. The surface to be exposed (10 cm2) was delimited by gluing an aluminum ring onto the clipped area with cyanoacrylate glue (Loctite, Senlis, France). The experiments were performed with [14C]-BPA dissolved in acetone (4 mg BPA/ml, 50 μl/cm²). Individual doses were determined by weighing the syringe before and after each deposit. The acetone was allowed to evaporate and the ring was covered with a Tedlar® membrane for occlusion. The radiocarbon dose was about 0.7 MBq/kg (20 μCi/kg). Immediately after BPA application, the animals were placed in metabolism cages for urine and feces collection. Batches of 3–5 rats were euthanatized after different exposure times (1–30 h). At the end of the exposure period, the skin around the ring was washed with a swab humidified with ethanol to detect any leakage of BPA outside the treated site. Ethanol (500 μl) was introduced through the Tedlar® membrane to dissolve the remaining surface BPA. The membrane was then cut and the non-absorbed fraction of BPA at the application site removed. The skin was dried with cotton swabs, which were scintillation counted separately. The radioactivity content was measured in the following samples: plasma, exposed skin, skin around the exposure site, ring + membrane, swabs, urine, feces (for 24 and 30 h of exposure), and carcass.
Ex vivo percutaneous absorption of BPA in rats and humans
Ex vivo percutaneous absorption was assessed using static Franz diffusion cells with fresh full thickness or dermatomed rat skin or human skin samples. Rats were killed by intra-peritoneal injection of a lethal dose of sodium pentobarbital. The entire dorsal region was clipped and the subcutaneous tissue carefully removed. Human skin samples were obtained from patients undergoing plastic surgery. The skin was cut into circular sections (1.76 cm²) and placed in diffusion cells with the stratum corneum facing up. Skin thickness was measured with a plate thickness gauge (Prost-Bourillon, Lunéville, France). Diffusion cells were maintained at a temperature of 36°C with a circulating water bath, which yielded a skin surface temperature of 32 ± 1°C. The dermis side was kept in contact with the receptor fluid (RPMI, 2% BSA, 1% penicillin/streptomycin), which had been previously filtered through a sterile Millex® 0.22-μm-pore-size filter and degassed with a vacuum pump. The solubility of BPA in this receptor fluid is at least 300 μg/ml. [14C]-BPA in acetone (4 mg BPA/ml) was applied to the skin (50 μl/cm² and 200 μg/cm²) for 24 h. An aliquot of receptor fluid was collected regularly over the 24-h period with an automatic fraction collector (Gilson FC 204, Middleton, WI, USA). The volume of receptor fluid was maintained at a constant level by adding the same quantity of fresh receptor fluid to the cells automatically. At the end of the experiment, the unabsorbed dose of [14C]-BPA was removed from the skin surface with ethanol and dry cotton swabs, as described for the in vivo experiments. The skin was digested in an 80% ethanol solution of KOH (1.5 M). Total radioactivity in the various fractions was determined by direct liquid scintillation counting.
The integrity of each skin sample was assessed before performing permeation experiments by the measurement of trans-epidermal water loss (TEWL), one of the three methods recommended by the OECD (guidance 28, 2004). TEWL measurements have the advantage that no solutions have to be added to perform the barrier integrity test other than those used in the permeation experiments. Samples with a TEWL value higher than 13 g/m²/h were discarded. The rate of metabolism of water-soluble tetrazolium salt (WST) into formazan was used to evaluate cellular viability. A non-viable skin control was obtained by freezing samples for 1 h at −20°C.
HPLC analysis of urinary BPA by fluorescence
BPA and its metabolites (glucuronides and/or sulfonides) were analyzed by HPLC according to the method developed by Matsumoto et al. (2003). Urine (100 μl) was buffered with 50 μl of 0.01 M sodium acetate buffer (pH 5.0) and hydrolyzed enzymatically with 5 μl of β-glucuronidase/sulfatase using Helix pomatia (Merck, Darmstadt, Germany), for 18 h at 37°C. After hydrolysis, 445 μl of acetonitrile was added to the hydrolysate and it was placed for 15 min at −20°C. After centrifugation for 10 min at 20,000g, 300 μl of the supernatant was transferred to a new tube and 200 μl of 0.01 M sodium acetate buffer (pH 2.7) was added. The solution (100 μl) was injected onto the HPLC system.
The HPLC system (Varian) consisted of a 9012 pump system operating at 1.0 ml/min flowrate; the mobile phase was prepared by mixing 0.01 M sodium acetate buffer (pH 2.7) and acetonitrile (65:35) in the isocratic mode; a 410 autosampler, which injected 100 μl of the processed sample into the system; a Waters Spherisorb® ODS2 5-μm column (4.6 mm inner diameter × 250 mm length); a Prostar 363 fluorescence detector (excitation 275 nm/emission 300 nm).
Data reporting
Statistic analysis was performed with Statgraphics software (Sigma Plus, Toulouse, France). Values were expressed as mean ± SD. ANOVA followed by a Dunett’s test was used to determine statistical significance. The level of significance was set at P < 0.05.
The percentage in vivo absorption was calculated using the radioactivity content in excreta and carcass, as follows:
Percentage penetration was calculated using the radioactivity content in excreta, carcass, and exposed skin area:
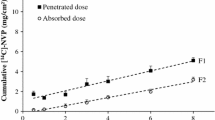
Absorption flux and penetration flux, expressed in μg/h/cm² were calculated from the slope of the cumulative curve of the absorbed and penetrated doses as a function of time (Fig. 1), respectively.
In vivo cumulative percutaneous penetration and absorption of [14C]-BPA in male Sprague–Dawley rats. Batches of rats were euthanatized at different times after a topical exposure to [14C]-BPA under occlusion. [14C]-BPA was dissolved at 4 mg/ml in acetone, 50 μl/cm², corresponding to a dose of 200 μg of BPA/cm² were applied to the skin over a 10 cm² area. Acetone was allowed to evaporate. Values are expressed as mean ± SD. Cumulative penetration (filled square) and absorption (open circle) versus time are plotted. Penetration flux: F1 (μg/cm²/h) = 2.6 (±0.2), r = 0.97. Absorption flux: F2 (μg/cm²/h) = 2.5 (±0.2), r = 0.98. Tlag is not significantly different from 0 for either curve
The lag time (Tlag) was calculated as the intercept of the steady state portion of the curve on the time axis.
The percentage of the absorbed dose ex vivo was calculated from the radioactivity content detected in the receptor fluid:
The absorption flux F max, expressed in μg/cm²/h, is the maximum absorption flux determined during exposure.
Estimation of the absorbed dose in the workplace:
where Q is the daily absorbed quantity (μg/kg), F the absorption flux (μg/cm²/h), T the exposure time (h), S the exposed surface (cm²), and M the body weight (kg).
Results
Mass balance of [14C]-BPA after intravenous administration
The [14C] mass balance after a single intravenous administration of different doses of [14C]-BPA is presented in Table 1. Regardless of the dose administered, the percentages of [14C] excreted in feces and urine were not significantly different, indicating no saturation of the excretion pathway. The total recovery was in the range 90–101% of the administered dose. The apparent urinary excretion half-life calculated from the 50 and 500 μg/kg doses was 9.9 ± 2.1 h.
[14C] was predominantly excreted in feces, which accounted for about 63–75% of the excretion over the 72-h period. Within this period, urinary excretion accounted for about 12–22% of the total excreted [14C] and occurred essentially in the first 24 h after administration. After this period, [14C] was principally excreted in feces.
In vivo percutaneous absorption of BPA
The cumulative percutaneous absorption and penetration of [14C]-BPA are shown in Fig. 1. The absorbed (Eq. 1) and penetrated (Eq. 2) doses increased linearly with exposure time. The penetration and absorption fluxes, calculated from the cumulative curves of penetrated and absorbed doses as a function of time, were identical (2.5 ± 0.2 μg/cm²/h). Penetration flux was maximal after 1 h of exposure. The calculated lag times were not significantly different from zero.
BPA penetrated rapidly into the skin (Table 2). Skin content was not significantly different throughout exposure time (mean value was 31 ± 10 μg/cm²), indicating no accumulation of BPA in the skin. [14C] excreted in feces was three- to sixfold higher than in urine for an exposition time of 24 or 30 h. The total recovery was in the range of 90–100% of the administered dose.
The skin BPA content was measured either at the end of an 8-h percutaneous exposure or 64 h after an 8-h exposure was 33.0 ± 6.0 and 6.2 ± 2.2 μg/cm², respectively. Over this time period, the absorbed dose increased from 25 ± 8 μg/cm² (8 h exposure) to 46 ± 7 μg/cm² (64 h later).
Ex vivo percutaneous absorption of BPA
Effect on integrity and viability
The effect of BPA on the integrity and viability of the skin was evaluated (Table 3). After exposure to 200 μg/cm² BPA for 24 h, viability and integrity of the tested samples were not significantly different from control samples (viable skin without BPA).
Cutaneous metabolism of BPA
BPA may be metabolized as it passes through the skin, and the extent of this metabolism was estimated by measuring BPA metabolites in the fluid receptor after 24-h exposure to BPA (200 μg/cm²) on fresh dermatomed rat and human skin samples. For both human and rat skin, unmodified BPA accounted for more than 97% of the radioactivity detected in the receptor fluid (Table 4).
Human/rat comparison
Results from humans and rats were compared for percutaneous exposure to [14C]-BPA on dermatomed skin samples (thickness about 0.4 mm) (Table 5). The percutaneous absorption flux (F max) measured on human skin was about tenfold lower than the flux measured on rat skin.
Discussion
Bisphenol A is a widely used monomer with established estrogenic properties. The objective of this study was to determine in vivo and ex vivo percutaneous absorption fluxes for BPA in rats and to see how these results could be extrapolated to humans.
After intravenous administration of increasing doses of [14C]-BPA (from 10 to 1,000 μg/kg), the mass balance showed that [14C] was predominantly excreted in feces (63–74% of the administered dose). From the five doses tested, no significant difference was found in terms of excreted quantities of [14C] in feces and urine as a function of the doses administered. Thus, there was no saturation of the BPA excretion pathway. The urinary excretion half-life calculated from the kinetics of urinary elimination was 9.9 ± 2.1 h. Urinary excretion accounted for between 12 and 22% of the administered dose and occurred principally within the first 24 h post-dosing. Previous reports had also indicated that about 80–90% of the urinary [14C] was excreted during the first 24 h (Kurebayashi et al. 2003). After intravenous administration of [14C]-BPA in rats, 78 and 13% of the administered dose were recovered in feces and urine, respectively, within the first 48 h.
Using the data from rats, illustrating cumulative curves of penetrated and absorbed doses over time, the percutaneous fluxes for penetration and absorption were found to be identical (2.5 ± 0.2 μg/cm²/h). Moreover, the skin content did not change significantly between 1 and 30 h, or at any time during exposure, with a mean value of 31 ± 10 μg/cm². These results indicated that throughout exposure, there was no accumulation of BPA in the skin. Thus, under the conditions used in this study, BPA penetrated rapidly into the skin and the maximal penetration flux was obtained after 1 h of exposure. Sixty-four hours after an 8-h exposure, the skin content decreased from 33 to 6 μg/cm². Over the same time period, the calculated absorbed dose increased from 25 to 46 μg/cm². These results showed that the BPA present in skin at the end of exposure is available for diffusion and absorption. Thus, the skin constituted a reservoir for BPA. This skin reservoir effect after the end of exposure to BPA may explain the increase in the urinary half-life for the excretion of 14C compared with the i.v. route (28 and 10 h, respectively). It has also been demonstrated that the skin acts as a reservoir for several other molecules such as corticosteroids (Vickers 1963) and lipophilic compounds (Kemppainen et al. 1992; Payan et al. 2001). In particular, a two-fold increase in urinary T1/2 was found for pyrene when comparing i.v. and trans-cutaneous routes (Payan et al. 2008).
In order to examine a possible toxic effect of BPA on the skin, viability and physical integrity of skin samples were measured ex vivo after 24-h exposure to 200 μg/cm² BPA. Trans-epidermal water loss (TEWL), which measures the skin’s barrier function, and WST colorimetric assay for cell viability, which reflects mitochondrial activity, were not significantly different in test and control samples. At the studied dose, BPA was therefore not considered to be cytotoxic for the skin and did not affect the skin’s integrity.
Prior to their passage through the skin, some molecules have to be metabolized (Hewitt et al. 2000; Payan et al. 2001, 2008, 2009; Zhang et al. 2009). The receptor fluids of rat and human viable skin exposed for 24 h to BPA were analyzed by HPLC to establish their metabolic profiles. Under our conditions, for both rats and humans, unmodified BPA accounted for more than 97% of the radioactivity found in the receptor fluid, suggesting that BPA was not metabolized during its skin transfer. In contrast, Zalko et al. (2010) concluded in a recent study that BPA was efficiently absorbed and metabolized in short-term culture of viable pig ear skin and human explants. The major metabolites produced were BPA mono-glucuronide and BPA mono-sulfate. However, skin viability did not significantly modify the absorption rate of BPA. Indeed, the percentages of radioactivity recovered in the culture media were 65.3 ± 8.2% and 58.1 ± 3.6% respectively, for viable and non-viable pig skin explants. These findings were confirmed by the comparison of absorption kinetics carried out ex vivo on rat skin using fresh (viable) and frozen (non-viable and no metabolic activity) skin samples. The F max values measured, 1.7 ± 0.5 and 2.1 ± 0.4 μg/cm²/h for viable and non-viable skin respectively, were not significantly different.
Using frozen human dermatomed skin samples about 400 μm thick, the measured F max was about tenfold lower than that for the rat under the same exposure conditions (1.48 ± 0.41 μg/cm²/h for rat vs. 0.12 ± 0.09 μg/cm²/h for human). A similar difference in skin permeability between human and rat was previously reported for the percutaneous passage of BPA diglycidyl ether (BADGE) on dermatomed fresh skin (Boogaard et al. 2000). For BPA, a significant level of intra- and inter-individual variability was observed with human skin samples, varying up to tenfold. In a multi-center comparative study, conducted in vitro with three different products on human skin samples, van de Sandt et al. (2004) showed a similar level of variation, which they attributed to differences in human skin samples.
In the rat, extrapolation of the ex vivo results obtained with dermatomed skin samples (about 500 μm of thickness) to model what happens in vivo may be possible. Indeed, the F max-measured ex vivo (1.48 μg/cm²/h) was relatively close to the in vivo value (2.5 μg/cm²/h). If we allow a similar extrapolation for human skin, it becomes possible to give an estimate of occupational cutaneous exposure to BPA. Considering a 1-h exposure over 2,000 cm², representing the forearms and the hands, (ECETOC 1993) and using the normally assumed 60 kg body weight, percutaneous absorbed BPA would be about 4 μg/kg/day (Eq. 4). In a recent study, Biedermann et al. (2010) evaluated a possible BPA transfer from thermal printing paper (containing 8–17 g/kg of BPA) to the forefinger and the middle finger. The obtained results lead the authors to estimate at 71 μg/day the potential exposure of a person repeatedly touching this type of paper for 10 h/day. These two results represent approximately 2–10% of the present TDI, but are not so far from the value for which adverse effects were observed in animal experiments. Evaporation from the skin may limit the degree of exposition in occupational exposures and act like a barrier against toxicants. In the case of bisphenol A, Biedermann et al. (2010) concluded that the BPA transferred to the forefinger and the middle finger could be ten times more if these fingers were wet or very greasy, suggesting that evaporation could enhance the BPA skin absorption. Experiment conducted ex vivo in the rats with or without occlusion showed that under our experimental conditions, there were no significant differences for the fluxes measured in both conditions (data not shown).
In conclusion, BPA penetrated rapidly into the skin and was not at all or only very slightly metabolized during its passage through the skin. In vivo and ex vivo absorption fluxes measured using rat skin were similar and thus permitted an extrapolation of human in vivo values based on ex vivo results obtained on dermatomed skin. The estimated in vivo value of 4 μg/kg/day is not so far from the dose for which adverse effects have been observed in some experiments in rats and mice. Thus, this value must be taken into account when evaluating risks related to BPA exposure, at least until more relevant results on the toxicity of BPA in humans are available.
Abbreviations
- BPA:
-
Bisphenol A
- CERHR:
-
Center for evaluation of risks to human reproduction
- EFSA:
-
European food safety authority
- HPLC:
-
High performance liquid chromatography
- LOAEL:
-
Lowest observed adverse effect level
- NTP:
-
National toxicology program
- TDI:
-
Tolerable daily intake
- TEWL:
-
Trans-epidermal water loss
References
Al-Hiyasat AS, Darmani H, Elbethiea AM (2002) Effects of bisphenol A on adult male mouse fertility. Eur J Oral Sci 110:163–167
Biedermann S, Tschudin P, Grob K (2010) Transfer of bisphenol A from thermal printer paper to the skin. Anal Bioannal Chem 398:571–576
Boogaard PJ, Denneman MA, Van Sittert NJ (2000) Dermal penetration and metabolism of five glycidyl ethers in human, rat and mouse skin. Xenobiotica 30:469–483
Burridge E (2003) Bisphenol A: product profile. Eur Chem News 17:14–20
Calafat AM, Kuklenyik Z, Reidy JA, Caudill SP, Ekong J, Needham LL (2005) Urinary concentrations of bisphenol A and 4-nonylphenol in a human reference population. Environ Health Perspect 113:391–395
Domoradzki JY, Pottenger LH, Thornton CM, Hansen SC, Card TL, Markham DA, Dryzga MD, Shiotsuka RN, Waechter JM Jr (2003) Metabolism and pharmacokinetics of bisphenol A (BPA) and the embryo-fetal distribution of BPA-monoglucuronide in CD Sprague–Dawley rats at three gestational stages. Toxicol Sci 76:21–34
ECETOC (1993) Percutaneous absorption. Monograph report no 20
EFSA Panel on food contact materials, enzymes, flavourings and processing aids (CEF) (2010) Scientific opinion on bisphenol A: evaluation of a study investigating its neurodevlopmental toxicity, review of recent scientific literature on its toxicity and advice on the Danish risk assessment of bisphenol A. EFSA J 8(9):1829 [110 pp]
Hewitt PG, Perkins J, Hotchkiss SA (2000) Metabolism of fluroxypyr, fluroxypyr methyl ester, and the herbicide fluroxypyr methylheptyl ester. I: during percutaneous absorption through fresh rat and human skin in vitro. Drug Metab Dispos 28:748–754
Kaddar N, Harthe C, Dechaud H, Mappus E, Pugeat M (2008) Cutaneous penetration of bisphenol A in pig skin. J Toxicol Environ Health 71:471–473
Kemppainen BW, Mehta M, Stafford R, Riley RT (1992) Effect of vehicle on skin penetration and retention of a lipophilic red tide toxin (PbTx-3). Toxicon 30:931–935
Kurebayashi H, Harada R, Stewart RK, Numata H, Ohno Y (2002) Disposition of low dose of bisphenol A in male and female cynomolgus monkeys. Toxicol Sci 68:32–42
Kurebayashi H, Betsui H, Ohno Y (2003) Disposition of low dose of [14C]-bisphenol A in male rats and its main biliary excretion as BPA glucuronide. Toxicol Sci 73:17–25
Lang IA, Galloway TS, Scarlett A, Henley WE, Depledge M, WallaceRB MelzerD (2008) Association of urinary bisphenol A concentration with medical disorders and laboratory abnormalities in adults. JAMA 300:1303–1310
Matsumoto A, Kunugita N, Kitagawa K, Isse T, Oyama T, Foureman GL, Morita M, Kawamoto T (2003) Bisphenol A levels in human urine. Environ Health Perspect 111:101–104
NTP-CEHRH monograph on the potential human reproductive and developmental effects of bisphenol A (2008) U.S. department of health and human services, NIH publication no 08-5994, p 321
Palanza P, Howdeshell KL, Parmigiani S, vom Saal FS (2002) Exposure to a low dose of bisphenol A during fetal life or in adulthood alters maternal behavior in mice. Environ Health Perspect 110:415–422
Payan JP, Marty JP, Beydon D, Boudry I, Ferrari E, Canel F, Grandclaude MC, Vincent CM (2001) In vivo and in vitro percutaneous absorption of [14C]di-N-butylphthalate in rat. Drug Metab Dispos 29:843–854
Payan JP, Lafontaine M, Simon P, Marquet F, Champmartin-Gendre C, Beydon D, Ferrari E (2008) In vivo and in vitro percutaneous absorption of [14]pyrene in Sprague–Dawley male rats: skin reservoir effect and consequence on urinary 1-OH pyrene excretion. Arch Toxicol 82:739–747
Payan JP, Lafontaine M, Simon P, Marquet F, Champmartin-Gendre C, Beydon D, Wathier L, Ferrari E (2009) 3-Hydroxybenzo(a)pyrene as a biomarker of dermal exposure to benzo(a)pyrene. Arch Toxicol 83:873–883
Rubin BS, Murray MK, Damassa DA, King JC, Soto AM (2001) Perinatal exposure to low doses of bisphenol A affects body weight, patterns of estrous cyclicity, and plasma LH levels. Environ Health Perspect 109:675–680
Sekizawa J (2008) Low-dose effects of bisphenol A: a serious threat to human health? J Toxicol Sci 33:389–403
Van de Sandt JJM, van Burgsteden JA, Cage S, Carmichael PL, Dick I, Kenyon S, Korinth K, Limasset JC, Maas WJM, Montomoli L, Nielsen JB, Payan JP, Robinson E, Sartorelli P, Schaller KH, Wilkinson SC, Williams F (2004) In vitro predictions of skin absorption of caffeine, testosterone and benzoic acid: a multi-centre comparison study. Regul Toxicol Pharmacol 39:271–281
Vickers C (1963) Existence of reservoir in the stratum corneum. Experimental proof. Arch Dermatol 88:21–23
Völkel W, Kiranoglu M, Fromme H (2008) Determination of free and total bisphenol A in human urine to assess daily uptake as a basis for a valid risk assessment. Toxicol Lett 179:155–162
Völkel W, Colnot T, Csanady GA, Filser JG, Dekant W (2002) Metabolism and kinetics of bisphenol A in humans at low doses following oral administration. Chem Res Toxicol 15:1281–1287
Vom Saal FS, Hughes C (2005) An extensive new literature concerning low-dose effect of bisphenol A shows the need for a new risk assessment. Environ Health Perspect 113:926–933
Zalko D, Jacques C, Duplan H, Bruel S, Perdu E (2010) Viable skin efficiently absorbs and metabolizes Bisphenol A. Chemosphere (in press)
Zhang Q, Grice JE, Wang G, Roberts MS (2009) Cutaneous metabolism in transdermal drug delivery. Curr Drug Metab 10:227–235
Acknowledgments
The authors would like to thank Pr. P. Maxant and Pr. P. Sibille for their invaluable help.
Open Access
This article is distributed under the terms of the Creative Commons Attribution Noncommercial License which permits any noncommercial use, distribution, and reproduction in any medium, provided the original author(s) and source are credited.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
Open Access This is an open access article distributed under the terms of the Creative Commons Attribution Noncommercial License (https://creativecommons.org/licenses/by-nc/2.0), which permits any noncommercial use, distribution, and reproduction in any medium, provided the original author(s) and source are credited.
About this article
Cite this article
Marquet, F., Payan, JP., Beydon, D. et al. In vivo and ex vivo percutaneous absorption of [14C]-bisphenol A in rats: a possible extrapolation to human absorption?. Arch Toxicol 85, 1035–1043 (2011). https://doi.org/10.1007/s00204-011-0651-z
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00204-011-0651-z