Abstract
Rationale
Chronic alcoholism is associated with mild to moderate cognitive impairment. Under certain conditions, impairment can be ameliorated by invoking compensatory processes.
Objective
To identify electrophysiological mechanisms of such compensation that would be required to resolve response conflict.
Methods
14 abstinent alcoholic men and 14 similarly aged control men performed a variation of the Eriksen flanker task during an electroencephalography (EEG) recording to examine whether alcoholics could achieve and maintain control-level performance and whether EEG markers could identify evidence for the action of compensatory processes in the alcoholics. Monitoring processes engaged following a response were indexed by the correct related negativity (CRN) and error related negativity (ERN), two medial–frontal negative event-related potentials.
Results
The alcoholics were able to perform at control levels on accuracy and reaction time (RT). Alcoholics generated larger ERN amplitudes following incorrect responses and larger CRNs following correct responses than controls. Both groups showed evidence of post-error slowing. Larger CRN amplitudes in the alcoholics were related to longer RTs. Also observed in the alcoholics was an association between smaller CRN amplitudes and length of sobriety, suggesting a normalization of monitoring activity with extended abstinence.
Conclusions
To the extent that greater amplitude of these electrophysiological markers of performance monitoring indexes greater resource allocation and performance compensation, the larger amplitudes observed in the alcoholic than control group support the view that elevated performance monitoring enables abstinent alcoholics to overcome response conflict, as was evident in their control-level performance.
Similar content being viewed by others
Introduction
Chronic alcoholics commonly exhibit mild to moderate deficits in selective components of cognition (Sullivan and Pfefferbaum 2005; Fein et al. 2006; Oscar-Berman and Marinkovic 2007). Despite such functional compromise, recent evidence suggests that alcoholics can perform certain otherwise impaired cognitive tasks at control levels by engaging compensatory processes (Pfefferbaum et al. 2001; De Rosa et al. 2004; Chanraud-Guillermo et al. 2009; Gilman et al. 2010). How these compensatory processes are invoked, however, remains unknown. A potential process in eliciting compensation is performance monitoring, which enables flexible actions in response to moment-to-moment changes in the environment that can result in conflict (Botvinick et al. 2001) or deviations from reward expectancies (Holroyd and Coles 2002) while performing a task. Detection of these unfavorable events is indexed by event-related potentials generated from the medial–frontal cortex (Falkenstein et al. 1991; Gehring et al. 1993; Botvinick et al. 2001; Holroyd and Coles 2002).
The error related negativity (ERN; also called error negativity) (Falkenstein et al. 1991; Gehring et al. 1993) is an event-related potential (ERP) generated over fronto-central electrode scalp sites within 100 ms of an incorrect response. Larger ERNs have been associated with fast-acting compensatory systems that are able to inhibit and correct an error as it occurs and with slower acting systems that prolong reaction time (RT) on trials following errors (Gehring et al. 1993). Although the ERN was originally thought to index detection of errors, this interpretation was expanded when a negative potential similar to the ERN, the correct related negativity (CRN) (Vidal et al. 1998), was observed during correct trials high in response conflict (Vidal et al. 2000).
Vidal et al. (2000) tested subjects using a Stroop-like task written in French. The colored word stimuli were “rouge” (red), “bleu” (blue), and “vert” (green). The stimulus words could be written in red, blue, or green, thus creating trials with congruent (for example, “rouge” written in red) and incongruent (for example, “rouge” written in blue) stimuli. Responses were determined by the color of these imperative stimuli (that is, a stimulus eliciting a natural or learned response). Subjects responded with their left hand to one of the colors, right hand to another color, and withheld a response to the third color. Error trials produced an ERN and correct trials generated a CRN. Despite the different behavioral outcomes, the ERN and CRN had the same latency, polarity, and topography, and both appeared to originate from the medial–frontal cortex, most likely from the anterior cingulate cortex (Vidal et al. 2000). These observations led to a reinterpretation of the function of these waveforms, whereby the ERN and the CRN reflect the detection of conflict following responses rather than overt errors (Carter et al. 1998; Gehring and Fencsik 2001). Even though these potentials originate from the same cortical areas and appear to have the same function, it is clear from the diverse findings in the literature that they are differentially sensitive to other underlying processes (Luu et al. 2000a, b; Bartholow et al. 2005; van Veen and Carter 2006; Hewig et al. 2007; Taylor et al. 2007; Segalowitz and Dywan 2009).
Conflict in choice reaction time can be elicited experimentally with compatibility paradigms, such as the Stroop (Stroop 1935), Eriksen (Eriksen and Eriksen 1974), and Simon (Craft and Simon 1970) tasks. Conflict in this context is thought to result from incompatible response representations being simultaneously activated during processing. The imperative stimuli are intended to have task relevant (color of the stimulus) and task irrelevant (position of the stimulus) dimensions. Both dimensions are processed in parallel along different neural paths. These tasks are usually designed so that the irrelevant dimension has the property of being processed in a more automatic manner (prepotent response route, such as reading a colored word), and thus the irrelevant path (e.g., saying the color of the word when they are incongruent) is activated faster than the task relevant route. Therefore, when the spatial position of the stimulus is compatible with the response position, both routes are activated and lead to the correct response; however, when the stimulus appears on the opposite side of the response, both the correct and incorrect response routes are activated. The co-activation of two potential responses results in conflict (Duncan-Johnson and Kopell 1981; Burle et al. 2002) and may be indexed by ERN and CRN amplitudes.
Herein, whether alcoholics engage in performance monitoring at normal levels was examined using scalp electrode recording while subjects performed a variation of the Eriksen flanker task (Gehring and Knight 2000). We tested the hypothesis that alcoholics engage in enhanced conflict monitoring marked by abnormally high ERN and CRN amplitudes to maintain normal performance levels relative to controls.
Materials and methods
Participants
We tested 14 abstinent alcoholic men and 14 similarly aged control men. All were right handed and had normal or corrected to normal vision. Written informed consent to participate in the study was given by all subjects after reading a detailed description of the procedures and having their questions answered. The protocol was approved by the Stanford University and SRI International institutional review boards, and was in accordance with the Declaration of Helsinki. All participants underwent medical and psychiatric screening that included a structured alcohol history (Pfefferbaum et al. 1988) and structured clinical interview [Structured Clinical Interview for Axis I DSM-IV Disorders (SCID)] (First et al. 1998). All alcoholic participants and none of the control subjects met the DSM-IV criteria for alcohol dependence. Alcoholics were between 1 and 4 months abstinent. Controls consumed significantly less alcohol during their lifetime than the alcoholic men. The control men were significantly more educated than the alcoholic men. There was no difference in socioeconomic status (SES) or National Adult Reading Test (NART) intelligence quotient (IQ) (Nelson 1982) scores between the groups. All 14 alcoholics endorsed having been arrested compared with only four controls (Pearson’s Chi-square < 0.000); the alcoholics were arrested 14.7 more times than the controls. Six alcoholics and four controls endorsed having either a mother or father with a drinking problem; none of the subjects endorsed both parents as having problems with alcohol. Thus, the groups did not differ in proportion of individuals with a positive history of alcoholism (Pearson’s Chi-square = 0.414). Demographics for both groups are in Table 1.
Behavioral task
The experimental paradigm, a flanker letter discrimination task (Gehring and Knight 2000), was programmed using E-Prime 2 software. Responses were collected on a Psychology Software Tools serial response box. Stimuli were presented on a 16 in. Dell UltraSharp monitor via an NVIDIA GeForce FX 5200 video card. Subjects were instructed to respond as quickly and accurately as possible. They were told not to dwell on their mistakes but rather maintain pace with the experiment. After achieving the performance criteria above 60% accuracy, the subjects completed five blocks of 96 trials each with rest periods in between the blocks. A trial was composed of a cue, fixation stimulus, imperative stimulus, and response (Fig. 1). A random inter trial interval between 400 and 800 ms was used. The cue, either the word “green” or “red” printed in black on white background, was presented at time zero for 200 ms. A centrally located fixation cross was presented after the cue for a random length of time between 400 and 800 ms. The imperative stimuli were composed of a centrally presented fixation stimulus and two letters, one letter on each side of the fixation, one colored red and the other green  The target letter was identified by the color cue. The subjects had as long as 1,000 ms to identify the target letter and respond. Subjects responded by pressing a left button with their left index finger when the target letter was an H or a right button press with their right index finger when the target letter was an S. Trials in which the target and flanker letter were the same
The target letter was identified by the color cue. The subjects had as long as 1,000 ms to identify the target letter and respond. Subjects responded by pressing a left button with their left index finger when the target letter was an H or a right button press with their right index finger when the target letter was an S. Trials in which the target and flanker letter were the same  were considered low-conflict stimuli (Ls). Trials in which the target letter was different from the flanker letter
were considered low-conflict stimuli (Ls). Trials in which the target letter was different from the flanker letter  were considered high-conflict stimuli (Hs). Trials in which the target letter was presented on the same side of the screen as the required response hand were considered low-conflict response (Lr). Trials in which the target letter appeared on the opposite side of the screen as the required response hand were considered high-conflict response (Hr) trials. Accordingly, for analysis, trials were segregated into four categories: LsLr, LsHr, HsLr, and HsHr. Pseudo-randomized presentation of stimuli generated 24 trials of each category type per block.
were considered high-conflict stimuli (Hs). Trials in which the target letter was presented on the same side of the screen as the required response hand were considered low-conflict response (Lr). Trials in which the target letter appeared on the opposite side of the screen as the required response hand were considered high-conflict response (Hr) trials. Accordingly, for analysis, trials were segregated into four categories: LsLr, LsHr, HsLr, and HsHr. Pseudo-randomized presentation of stimuli generated 24 trials of each category type per block.
Task description. Outline of the sequence of events for the four different experimental conditions: high stimulus conflict and high response conflict (HsHr), high stimulus conflict and low response conflict (HsLr), low stimulus conflict and high response conflict (LsHr), low stimulus conflict and low response conflict (LsLr)
EEG data collection
The electroencephalography (EEG) and evoked potential data were recorded from a Neuroscan 64-Channel Quick-Cap (El Paso, TX, USA). All electrodes were placed according to the international 10–20 electrode placement standard. Two external electro-oculogram electrodes were used to monitor vertical and horizontal eye movements. Raw data were acquired with Neuroscan Synamps 2 amplifiers at a sampling rate of 1 kHz.
Data analysis
Behavioral data were analyzed using E-Data Aid and Microsoft Excel. Accuracy and mean proportion correct were calculated over all blocks. The RT analysis involved calculating the average RT per condition (HsHr, HsLr, LsHr, LsLr, Error) across all blocks. Errors of omission were excluded from the RT analysis. Post-error RT was calculated by averaging RTs for all correct trials following an error and compared to the post-correct RT measure, which was the average of RTs for all correct trials following other correct trials.
EEG data were analyzed offline with Neuroscan 4.5 Scan software in the following order. All EEG channels were referenced offline to linked mastoids. Data were band-pass-filtered using a zero phase shift finite impulse response (FIR) filter between 0.3 and 30 Hz. Vertical and horizontal ocular artifacts were reduced using the ocular artifact reduction algorithms from Neuroscan 4.5. When present, the EKG noise reduction algorithm from Neuroscan 4.5 was used to reduce these artifacts. All data were then visually inspected and any epochs with remaining artifact (muscle, movement, etc.) were manually rejected. Because the N2 is observed following the stimulus presentation, this potential was time-locked to the stimulus (stimulus-locked). By contrast, the ERN and CRN occur following a response, so these potentials were time-locked to the response (response-locked). Stimulus-locked ERPs were epoched from 400 ms before to 800 ms following the stimulus. Response-locked ERP epochs spanned from 200 ms before to 600 ms following the response. To improve the visual presentation of the data, all epoched waveforms were then band-pass-filtered using a zero phase shift FIR filter between 1 and 12 Hz. The first half of the pre-stimulus interval served as baseline for the stimulus-locked potentials (−400 to −200 ms) while the response-locked potentials were baseline-corrected using the first half of the pre-response interval (−200 to −100 ms). All of the fronto-central potentials (N2, CRN, and ERN) were examined at electrode FCZ because that is the topographical location where the peaks reach maximum negative amplitude. The N2 and CRN amplitudes are given for each correct trial condition type (HsHr, HsLr, LsHr, LsLr). ERN values reflect the average of incorrect responses across these four conditions because too few trials were available to produce a reliable waveform for each condition. The time interval in which the average or peak values were extracted were determined by both published results and visual inspection of the individual waveforms. These intervals are specified for each potential in their respective results section.
Statistical analysis
RTs for correct trial types, CRN amplitudes, and N2 amplitudes and latencies were subjected to ANOVAs with stimulus conflict (Hs, Ls) and response conflict (Hr, Lr) as repeated measure factors and diagnosis (controls, alcoholics) as a between-groups factor. Because the number of years of education was significantly different between groups, it was used as a covariate in all the statistical analyses meeting the homogeneity of variance assumption. Univariate ANOVAs were used to investigate error trial RT and ERN amplitudes with diagnosis as a between-group factor and years of education as a covariate; however, because years of education did not correlate with all the dependent variables, we also report the results for each variable without using years of education as a covariate. Correlation analyses were conducted separately for the control and alcoholic groups. Pearson correlations were conducted between the amplitudes of the fronto-central negativities (ERN and CRN) and (1) demographic variables [age, SES, NART IQ, total lifetime alcohol consumed, and number of days abstinent], (2) accuracy, and (3) RT measures (RTs for error, correct, post-error, and post-correct trials). RTs for correct trials and the CRN amplitudes in the correlation analyses represent the average value across all condition types (HsHr, HsLr, LsHr, and LsLr).
Results
Behavior
Accuracy and error type count
The groups did not differ in the detection or matching accuracy (p = 0.332; without using years of education as a covariate p = 0.454). All subjects made more errors of omission than commission [F (1, 26) = 5.92; p = 0.022], but there were no group differences in the number of either type of error committed (p = 0.523; Table 2.) There was neither a significant difference between the number of response errors made with the right (S response) and the left (H response) hand (p = 0.093) nor a group difference in these measures (p = 0.977).
Reaction time
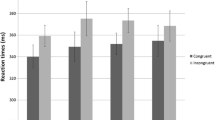
There were no group differences in response latency in any condition. No significant main effects of stimulus complexity, response congruence, or diagnosis (p > 0.197) were observed for RTs to correct responses. No significant interactions were observed between any of the factors (p > 0.113). RTs for incorrect responses also showed no effect of diagnosis (p > 0.440). RTs for all trial types are depicted in Fig. 2.
Reaction time (without years of education as covariate)
There were no group differences in RT (p = 0.173). A significant effect of stimulus complexity [F (1, 26) = 8.77; p = 0.006] and response congruence [F (1, 26) = 61.9; p < 0.000] were found. No interactions between factors were seen. RTs for incorrect responses showed no effect of diagnosis (p > 0.326). RTs for all trial types are depicted in Fig. 2.
Post-error slowing
Correct trials following error trials (post-error) were compared with correct trials following other correct trials (post-correct) to determine whether post-error slowing occurred. We found that RTs for correct trials following an incorrect response were marginally slower than correct trials following a correct response [F (1, 25) = 3.98; p = 0.057]; however, no group differences were observed in these measures (p = 0.521). See Table 3 for descriptive statistics.
Post-error slowing (without years of education as covariate)
Correct trials following error trials (post-error) were compared with correct trials following other correct trials (post-correct) to determine whether post-error slowing occurred. We found that RTs for correct trials following an incorrect response were slower than correct trials following a correct response [F (1, 26) = 47.4; p < 0.000]; however, no group differences were observed in these measures (p = 0.234). See Table 3 for descriptive statistics.
Evoked potentials
N2 amplitude and latency
The amplitude and latency of the frontal–central N2 were determined as the most negative peak between 250 and 465 ms following the stimulus presentation at electrode FCZ. Two repeated measures ANOVAs were conducted separately on amplitude and then on latency. Alcoholics generated similar amplitude and latency N2 potentials. Neither the diagnosis effect nor the effects of stimulus complexity or response congruency were significant for the amplitude or latency measures (p > 0.083) (N2 amplitude mean ± SE are found in Fig. 3, N2 waveforms are found in Fig. 4, and N2 latency mean ± SD are found in Table 4).
N2 amplitudes. Peak N2 values from electrode FCZ are plotted for the four condition types for correct responses. Error bars index standard error for each condition. Alcoholics produced similar amplitude N2 potentials to control group. This implies that similar stimulus processing occurred in both groups
N2 amplitude and latency (without years of education as covariate)
The amplitude and latency of the frontal–central N2 were determined as the most negative peak between 250 and 465 ms following the stimulus presentation at electrode FCZ. Two repeated measures ANOVAs were conducted separately on amplitude and then on latency. Alcoholics generated similar amplitude and latency N2 potentials. Neither the diagnosis effect nor the effects of stimulus complexity or response congruency were significant for the amplitude or latency measures (p > 0.205) (N2 amplitude mean ± SE are found in Fig. 3, N2 waveforms are found in Fig. 4, and N2 latency mean ± SD are found in Table 4).
CRN and ERN
Average CRN and ERN amplitudes were assessed at electrode FCZ between 20 and 120 ms following the response, which is the time interval within which these potentials reached their peak. A significant effect of diagnosis was revealed for the amplitude of the CRN [F(1, 24) = 12.08; p = 0.002]; here, the alcoholic group generated larger negative amplitude potentials for all condition types (HsHr, HsLr, LsHr, and LsLr) than the control group. After correcting for years of education, no main effect of stimulus complexity or response congruency and no interactions between the factors were observed (p > 0.146). The results of the univariate analysis on the ERN showed a significant effect of diagnosis [F(1, 24) = 5.83; p = 0.024] such that alcoholics thus also developed larger negative ERN amplitudes following incorrect responses than the controls (Figs. 5, 6).
a CRN amplitudes by condition. CRN mean amplitudes from electrode FCZ and their respective error bars, which index standard error values, are displayed for the four condition types for correct responses. Alcoholics generated significantly larger negative amplitudes than the controls suggesting that they engaged enhanced performance monitoring processes during all correct trial types. b CRN and ERN amplitudes. Mean CRN and ERN amplitudes from electrode FCZ are displayed with their corresponding standard error bars. CRN represents the mean amplitude across all correct trial types and the ERN represents the mean amplitude for all incorrect responses. Alcoholics generated significantly larger negative amplitude potentials for both correct and incorrect trials compared to the controls
a CRN waveforms. Response-locked CRN waveforms at electrode FCZ for correct responses are plotted for the four condition types (HsHr, HsLr, LsHr, LsLr) in dark blue (solid line) for controls and in red (dashed line) for alcoholics. Alcoholics produced larger CRN potentials for all trial types than control subjects. Note that the peak values represented in this figure do not correspond to the values reported in Fig. 5a as those are the average amplitude over a 100 ms time window. b CRN and ERN waveforms. Response-locked correct (left panel) and incorrect (right panel) waveforms are plotted at electrode FCZ in dark blue (solid line) for controls and in red (dashed line) for alcoholics. Waveforms were derived by averaging across all four trial types. Alcoholics compared to the controls produced significantly larger CRN amplitudes for all correct responses and larger ERN amplitudes to all incorrect responses. Note that the peak values represented in this figure do not correspond to the values reported in Fig. 5b as those are the average amplitude over a 100 ms time window
CRN and ERN (without years of education as covariate)
Average CRN and ERN amplitudes were assessed at electrode FCZ between 20 and 120 ms following the response, which is the time interval within which these potentials reached their peak. A significant effect of diagnosis was revealed for the amplitude of the CRN [F(1, 25) = 9.37; p = 0.005]; here, the alcoholic group generated larger negative amplitude potentials for all condition types (HsHr, HsLr, LsHr, and LsLr) than the control group. No main effect of stimulus complexity (p = 0.593) was observed. In contrast, an effect of response congruency [F(1, 25) = 10.48; p = 0.003) was found. No interactions between the factors were observed (p > 0.128). The results of the univariate analysis on the ERN showed a trend for an effect of diagnosis [F(1, 25) = 4.16; p = 0.052] such that alcoholics thus also developed larger negative ERN amplitudes following incorrect responses than the controls (Figs. 5, 6).
Correlations
CRN amplitudes for all correct trial types correlated with the number of days abstinent in alcoholics (r = 0.562, p = 0.045, N = 13], indicating that the CRN amplitude was less negative (closer to control values) with more days abstinent. Accuracy correlated with ERN amplitudes in alcoholics (r = −0.668, p = 0.012, N = 13). By contrast, in controls accuracy correlated with the amplitude of the CRN (r = 0.670, p = 0.009, N = 14). CRN amplitudes in alcoholics were in turn correlated with RTs for error (r = −0.611, p = 0.026, N = 13), correct (r = −0.644, p = 0.018, N = 13), post-error (r = −0.593, p = 0.033, N = 13), and post-correct trials (r = −0.657, p = 0.015, N = 13). The ERN did not correlate with any RT measures in alcoholics, and the RTs did not correlate with the CRN or ERN amplitudes in the control group.
Discussion
We tested the hypothesis that alcoholics are able to maintain performance at control levels at the cost of generating elevated levels of performance monitoring as indexed by abnormally enhanced ERN and CRN amplitudes. Consistent with these predictions, alcoholics performed the task at control levels and produced greater ERN and CRN amplitudes than the comparison group. We interpret these results as evidence of a compensatory response in the alcoholics.
Alcoholics and controls did not differ in error type, accuracy, or RT. Both groups produced ERNs following incorrect responses, but these negative potentials were larger for the alcoholics than the controls. Following correct responses, alcoholics also produced larger negative amplitude CRNs than controls. These electrical potentials index conflict monitoring processes poised to trigger control mechanisms that implement corrective or compensatory actions (Botvinick et al. 2001). Compensation following an error has traditionally been observed as a tendency to slow down (Rabbitt 1966; Gehring et al. 1993), referred to as post-error slowing. In our task, both the alcoholics and the controls showed a trend toward slowed RTs following errors, thus providing evidence that conflict monitoring control mechanisms were engaged in both groups. In addition, larger negative CRN amplitudes in the alcoholics were associated with longer RTs for all trial types (error, correct, post-error, and post-correct trials). While the study did not involve a direct test of impulsivity, it could be hypothesized that impulsivity would be reflected in a speed/accuracy trade-off in the reaction time task with high levels of impulsivity being associated with faster speed and lower accuracy. We did not find evidence of such a speed/accuracy trade-off in this sample of alcoholics; however, the enhanced processing seemed to have a secondary effect of slowing down responding, thereby enhancing accuracy. Furthermore, we found that CRN amplitudes were smaller in alcoholics with more days abstinent. Given this correlation, we speculate that alcohol dependence contributes to the enhanced performance monitoring activity, and that with sustained sobriety the need for enhanced performance monitoring is diminished.
Additional support for our interpretation that these enhanced evoked potentials thought to arise from medial–frontal brain regions (Niki and Watanabe 1979; Gehring et al. 1993; Dehaene et al. 1994; Kiehl et al. 2000; Carter and van Veen 2007), reflect an elicited compensatory mechanism in alcoholics, derives from the observation that larger negative ERN amplitudes in alcoholics were associated with greater accuracy. While, the correlational nature of the data require this interpretation to be made cautiously, it is however, supportive of evidence from other studies suggesting that the ERN is enhanced when accuracy is emphasized over speeded responses (Falkenstein et al. 1991; Gehring et al. 1993) and with the finding that the ERN increases as the certitude that an error was committed also increases (Dehaene et al. 1994; Luu et al. 2000a, b). Also consistent with our interpretation is the observation that the ERN increases with learning (Holroyd and Coles 2002). This electrical potential is thought to reflect the development of an internal representation of the correct response so that a greater mismatch exists between the representation of the correct and incorrect response (Holroyd and Coles 2002), thereby increasing the likelihood of performing the task accurately.
In the controls, accuracy was not related to the ERN but rather to the CRN. Larger negative amplitude CRNs were associated with lower accuracy in this comparison group, which is in stark contrast to the effects seen in the alcoholics. The ERN was not associated with any behavioral measures in the controls.
The fronto-central N2 has also been implicated in conflict detection associated with performance monitoring. This potential, which peaks between 250 and 350 ms after the onset of an imperative stimulus and before a response is made, may reflect the detection of conflict involved in stimulus evaluation or target detection because it occurs at such an early stage in processing (Zhang et al. 1999); however, the functional role of the N2 in conflict detection and performance monitoring is still uncertain due to inconsistent findings (van Veen et al. 2001; Donkers and van Boxtel 2004; Bartholow et al. 2005). We found no differences in the fronto-central N2 between conditions or between groups suggesting that this potential, at least when elicited by our task, was not related to compensatory mechanisms triggered by response monitoring.
Fein et al. studied a different aspect of performance monitoring in treatment naïve alcoholics using the balloon analogue risk task (BART) (Fein and Chang 2008). Instead of examining the ERN, which occurs following a response as done in the present study, Fein and colleagues focused their analysis on the feedback-ERN (F-ERN). The F-ERN is elicited by tasks in which subjects use positive and negative feedback to evaluate their responses and occurs approximately 250 ms following the feedback stimuli (Holroyd et al. 2004; Nieuwenhuis et al. 2004; Hajcak et al. 2006). They found that smaller F-ERN amplitudes were associated with a greater family history density of alcohol problems. Nonetheless, F-ERN amplitudes were not related to performance or the amount of money lost on the BART. Their results suggest that genetic vulnerability toward developing alcoholism may be related to decreased sensitivity to negative feedback. The fact that these findings appear to point to a deficit in performance monitoring in alcoholics is at first glance in opposition to our findings; however, it has been argued that the ERN and F-ERN do not represent the same component process (Gehring and Willoughby 2002). In fact, Holroyd and Coles (2002) found that while the ERN increased with learning, the F-ERN decreased with learning (Holroyd and Coles 2002). Furthermore, even when it has been argued that the ERN and the F-ERN are manifestations of the same underlying process (Nieuwenhuis et al. 2004), the relative or subjective relevance of an action moderates the amplitude of the ERN (Luu et al. 2000a, b; Hajcak et al. 2005; Hewig et al. 2007). In our task, the relevance of the responses was not manipulated. Therefore, it is possible that when the emotional or motivational state of alcoholics is affected, as it could potentially be affected in gambling tasks, a deficit may become evident; however, our data do not address the role of motivation or affect on behavioral or electrophysiological performance.
Although we did not screen subjects for axis II symptomatology, all 14 members of the alcoholic cohort endorsed having been arrested in the past, compared to only four controls. The significant difference in this measure between groups, suggests that the alcoholic group was more likely to engage in excessive risk taking behavior, which is common among individuals with conduct and antisocial personality disorder. Thus, we cannot rule out the possibility that the enhanced ERN/CRN amplitudes observed in the alcoholics may have been due to a pre-morbid condition that improved with alcohol rehabilitation treatment.
Other studies report that alcoholics show impaired cognitive efficiency, with reduced ability to attend to relevant information while identifying and ignoring irrelevant information (Nixon et al. 1998). Response monitoring potentials are hypothesized to reflect the development of an internal representation of a correct response leading to a greater mismatch between the representation of correct and incorrect responses (Holroyd and Coles 2002). It is thus possible that enhanced performance monitoring could lead to increased cognitive efficiency, and that discrepancies in the literature regarding cognitive deficits in alcoholics may derive from the varying amount of influence that response monitoring processes have on specific tasks. For example, choice reaction time tasks with large response monitoring effects show little to no difference in performance between controls and alcoholics. By contrast, alcoholics may demonstrate impairment on more complex tasks such as the Sternberg task, in which response monitoring processes are less able to influence behavior.
Several factors may limit whether our interpretation can generalize to other samples of alcoholics. Although we attempted to recruit both men and women, we were not successful in identifying a sufficient number of alcoholic women who met our inclusion criteria. Therefore, we only included men in this study and are not able to generalize our results to alcoholic women. A larger sample size may provide the opportunity to identify sources of variance and heterogeneity in alcoholics that could explain differences in response monitoring. Alcoholics in our study compared with alcoholics in other studies (Pfefferbaum et al. 1992; Colrain et al. 2009) were relatively young, had lower amounts of lifetime alcohol consumption, and therefore were not as severely impaired. Also, the range of sobriety in our study was limited. Perhaps with longer sobriety, other compensatory mechanisms may be invoked, as hinted by the negative correlations observed herein between lower CRN and longer sobriety.
Together, our results suggest that abstinent alcoholics are able to achieve control-level behavioral performance by generating enhanced performance monitoring activity which in turn engages compensatory mechanisms that slow down reaction times and increase the likelihood of making appropriate responses in later trials. A substantial literature based on surface electrode recordings in humans (Krigolson and Holroyd 2007; Gentsch et al. 2009; Hochman et al. 2009) and intracranial recordings in primates (Niki and Watanabe 1979) provide evidence for the medial–frontal cortex as the principal neural generator of the ERN. The medial–frontal cortex is also a major node in the default mode network (Raichle et al. 2001; Greicius et al. 2003), which exhibits greater activity when at rest than when engaged in a task and is proposed to enable introspection and self-awareness (Raichle and Snyder 2007). This overlap in function of the medial–frontal cortex leads to the speculation that compromised error monitoring could be related to damping of the default mode network in its capacity as a monitor of personal activity.
To the extent that greater amplitude of these electrophysiological markers of performance monitoring indexes greater resource allocation and performance compensation, the larger amplitudes observed in the alcoholic than control group support the view that elevated performance monitoring enables abstinent alcoholics to overcome response conflict, as was evident in their control-level performance. Thus, an implication of these results is that alcoholics who have maintained some reserve capacity are able to engage compensatory processing to achieve normal performance levels.
References
Bartholow BD, Pearson MA et al (2005) Strategic control and medial frontal negativity: beyond errors and response conflict. Psychophysiology 42(1):33–42
Botvinick MM, Braver TS et al (2001) Conflict monitoring and cognitive control. Psychol Rev 108(3):624–652
Burle B, Possamai CA et al (2002) Executive control in the Simon effect: an electromyographic and distributional analysis. Psychol Res 66(4):324–336
Carter CS, van Veen V (2007) Anterior cingulate cortex and conflict detection: an update of theory and data. Cogn Affect Behav Neurosci 7(4):367–379
Carter CS, Braver TS et al (1998) Anterior cingulate cortex, error detection, and the online monitoring of performance. Science 280(5364):747–749
Chanraud-Guillermo S, Andoh J et al (2009) Imaging of language-related brain regions in detoxified alcoholics. Alcohol Clin Exp Res 33(6):977–984
Colrain IM, Crowley KE et al (2009) The impact of alcoholism on sleep evoked Delta frequency responses. Biol Psychiatry 66(2):177–184
Craft JL, Simon JR (1970) Processing symbolic information from a visual display: interference from an irrelevant directional cue. J Exp Psychol 83(3):415–420
De Rosa E, Desmond JE et al (2004) The human basal forebrain integrates the old and the new. Neuron 41(5):825–837
Dehaene S, Posner MI et al (1994) Localization of a neural system for error detection and compensation. Psychol Sci 5(5):303–305
Donkers FC, van Boxtel GJ (2004) The N2 in go/no-go tasks reflects conflict monitoring not response inhibition. Brain Cogn 56(2):165–176
Duncan-Johnson CC, Kopell BS (1981) The Stroop effect: brain potentials localize the source of interference. Science 214(4523):938–940
Eriksen BA, Eriksen CW (1974) Effects of noise letters upon the identification of target letter in a nonsearch task. Percept Psychophys 16(1):143–149
Falkenstein M, Hohnsbein J et al (1991) Effects of crossmodal divided attention on late ERP components: II. Error processing in choice reaction tasks. Electroencephalogr Clin Neurophysiol 78(6):447–455
Fein G, Chang M (2008) Smaller feedback ERN amplitudes during the BART are associated with a greater family history density of alcohol problems in treatment-naive alcoholics. Drug Alcohol Depend 92(1–3):141–148
Fein G, Torres J et al (2006) Cognitive performance in long-term abstinent alcoholic individuals. Alcohol Clin Exp Res 30(9):1538–1544
First M, Spitzer R et al (1998) Structured clinical interview for DSM-IV Axis I disorders (SCID) version 2.0. New York State Psychiatric Institute, New York
Gehring WJ, Goss B et al (1993) A neural system for error detection and compensation. Psychol Sci 4:385–390
Gehring WJ, Knight RT (2000) Prefrontal-cingulate interactions in action monitoring. Nat Neurosci 3(5):516–520
Gehring WJ, Fencsik DE (2001) Functions of the medial frontal cortex in the processing of conflict and errors. J Neurosci 21(23):9430–9437
Gehring WJ, Willoughby AR (2002) The medial frontal cortex and the rapid processing of monetary gains and losses. Science 295(5563):2279–2282
Gentsch A, Ullsperger P et al (2009) Dissociable medial frontal negativities from a common monitoring system for self- and externally caused failure of goal achievement. Neuroimage 47(4):2023–2030
Gilman JM, Davis MB et al (2010) Greater activation in left hemisphere language-related regions during simple judgment tasks among substance-dependent patients in treatment for alcoholism. Alcohol Clin Exp Res 34(2):331–341
Greicius MD, Krasnow B et al (2003) Functional connectivity in the resting brain: a network analysis of the default mode hypothesis. Proc Natl Acad Sci USA 100(1):253–258
Hajcak G, Moser JS et al (2005) On the ERN and the significance of errors. Psychophysiology 42(2):151–160
Hajcak G, Moser JS et al (2006) The feedback-related negativity reflects the binary evaluation of good versus bad outcomes. Biol Psychol 71(2):148–154
Hewig J, Trippe R et al (2007) Decision-making in Blackjack: an electrophysiological analysis. Cereb Cortex 17(4):865–877
Hochman EY, Eviatar Z et al (2009) Source localization of error negativity: additional source for corrected errors. NeuroReport 20(13):1144–1148
Holroyd CB, Coles MG (2002) The neural basis of human error processing: reinforcement learning, dopamine, and the error-related negativity. Psychol Rev 109(4):679–709
Holroyd CB, Larsen JT et al (2004) Context dependence of the event-related brain potential associated with reward and punishment. Psychophysiology 41(2):245–253
Kiehl KA, Liddle PF et al (2000) Error processing and the rostral anterior cingulate: an event-related fMRI study. Psychophysiology 37(2):216–223
Krigolson OE, Holroyd CB (2007) Predictive information and error processing: the role of medial–frontal cortex during motor control. Psychophysiology 44(4):586–595
Luu P, Collins P et al (2000a) Mood, personality, and self-monitoring: negative affect and emotionality in relation to frontal lobe mechanisms of error monitoring. J Exp Psychol Gen 129(1):43–60
Luu P, Flaisch T et al (2000b) Medial frontal cortex in action monitoring. J Neurosci 20(1):464–469
Nelson HE (1982) National adult reading test windsor. NFER-Nelson, UK
Nieuwenhuis S, Yeung N et al (2004) Sensitivity of electrophysiological activity from medial frontal cortex to utilitarian and performance feedback. Cereb Cortex 14(7):741–747
Niki H, Watanabe M (1979) Prefrontal and cingulate unit activity during timing behavior in the monkey. Brain Res 171(2):213–224
Nixon SJ, Paul R et al (1998) Cognitive efficiency in alcoholics and polysubstance abusers. Alcohol Clin Exp Res 22(7):1414–1420
Oscar-Berman M, Marinkovic K (2007) Alcohol: effects on neurobehavioral functions and the brain. Neuropsychol Rev 17(3):239–257
Pfefferbaum A, Rosenbloom M et al (1988) Brain CT changes in alcoholics: effects of age and alcohol consumption. Alcohol Clin Exp Res 12(1):81–87
Pfefferbaum A, Lim KO et al (1992) Brain gray and white matter volume loss accelerates with aging in chronic alcoholics: a quantitative MRI study. Alcohol Clin Exp Res 16(6):1078–1089
Pfefferbaum A, Desmond JE et al (2001) Reorganization of frontal systems used by alcoholics for spatial working memory: an fMRI study. Neuroimage 14(1 Pt 1):7–20
Rabbitt PM (1966) Errors and error correction in choice-response tasks. J Exp Psychol 71(2):264–272
Raichle ME, Snyder AZ (2007) A default mode of brain function: a brief history of an evolving idea. Neuroimage 37(4):1083–1090, discussion 1097-1089
Raichle ME, MacLeod AM et al (2001) A default mode of brain function. Proc Natl Acad Sci USA 98(2):676–682
Segalowitz SJ, Dywan J (2009) Individual differences and developmental change in the ERN response: implications for models of ACC function. Psychol Res 73(6):857–870
Stroop JR (1935) Studies of interference in serial verbal reactions. J Exp Psychol 18(6):643–662
Sullivan EV, Pfefferbaum A (2005) Neurocircuitry in alcoholism: a substrate of disruption and repair. Psychopharmacology (Berl) 180(4):583–594
Taylor SF, Stern ER et al (2007) Neural systems for error monitoring: recent findings and theoretical perspectives. Neuroscientist 13(2):160–172
van Veen V, Carter CS (2006) Error detection, correction, and prevention in the brain: a brief review of data and theories. Clin EEG Neurosci 37(4):330–335
van Veen V, Cohen JD et al (2001) Anterior cingulate cortex, conflict monitoring, and levels of processing. Neuroimage 14(6):1302–1308
Vidal F, Hasbroucq T et al (1998) Neurophysiological indices of errors in human: is the error negativity specific of errors? Travaux Scientifiques des Chercheurs du Service de Sante des Armees 0(19):165–166
Vidal F, Hasbroucq T et al (2000) Is the ‘error negativity’ specific to errors? Biol Psychol 51(2–3):109–128
Zhang HH, Zhang J et al (1999) A parallel distributed processing model of stimulus–stimulus and stimulus–response compatibility. Cogn Psychol 38(3):386–432
Acknowledgments
The study was supported by funds granted from the NIAAA AA005965 (minority supplement to MP), AA017168, and AA014211.
Disclosure/conflict of interest
IMC has received grant support from the National Institutes for Health, Ventus Medical Inc., and ApniCure Inc. for work unrelated to that presented in the submitted manuscript. None of the remaining authors had any financial or other conflict with the work reported herein.
Open Access
This article is distributed under the terms of the Creative Commons Attribution Noncommercial License which permits any noncommercial use, distribution, and reproduction in any medium, provided the original author(s) and source are credited.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
Open Access This is an open access article distributed under the terms of the Creative Commons Attribution Noncommercial License (https://creativecommons.org/licenses/by-nc/2.0), which permits any noncommercial use, distribution, and reproduction in any medium, provided the original author(s) and source are credited.
About this article
Cite this article
Padilla, M.L., Colrain, I.M., Sullivan, E.V. et al. Electrophysiological evidence of enhanced performance monitoring in recently abstinent alcoholic men. Psychopharmacology 213, 81–91 (2011). https://doi.org/10.1007/s00213-010-2018-1
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00213-010-2018-1