Abstract
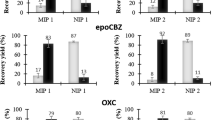
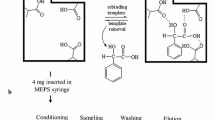
Cotinine, the main metabolite of nicotine in human body, is widely used as a biomarker for assessment of direct or passive exposure to tobacco smoke. A method for molecularly imprinted solid-phase extraction (MISPE) of cotinine from human urine has been investigated. The molecularly imprinted polymer (MIP) with good selectivity and affinity for cotinine was synthesized using cotinine as the template molecule, methacrylic acid as the functional monomer, and ethylene glycol dimethacrylate as the cross-linker. The imprinted polymer was evaluated for use as a SPE sorbent, in tests with aqueous standards, by comparing recovery data obtained using the imprinted form of the polymer and a non-imprinted form (NIP). Extraction from the aqueous solutions resulted in more than 80% recovery. A range of linearity for cotinine between 0.05 and 5 μg mL−1 was obtained by loading 1 mL blank urine samples spiked with cotinine at different concentrations in acetate buffer of pH 9.0, and by using double basic washing and acidic elution. The intra-day coefficient of variation (CV) was below 7% and inter-day CV was below 10%. This investigation has provided a reliable MISPE–HPLC method for determination of cotinine in human urine from both active smokers and passive smokers.







Similar content being viewed by others
References
Dhar P (2004) J Pharm Biomed Anal 35:155–168
Doctor PB, Gokani VN, Kulkarni PK, Parikh JR, Saiyed HN (2004) J Chromatogr B 802:323–328
Smith RF, Mather HM, Ellard GA (1998) Clin Chem 44:275–281
Hariharan M, VanNoord T (1991) Clin Chem 37:1276–1280
McAdams SA, Cordeiro ML (1993) J Chromatogr B 615:148–153
James H, Tizabi Y, Taylor R (1998) J Chromatogr B 708:87–93
Oddoze C, Pauli AM, Pastor J (1998) J Chromatogr B 708:95–101
Xu AS, Peng LL, Havel JA, Petersen ME, Fiene JA, Hulse JD (1996) J Chromatogr B 682:249–261
Ubbink JB, Lagendijk J, Vermaak WHH (1993) J Chromatogr B 620:254–259
Knight GJ, Palomaki GE, Lea GE, Haddow JE (1989) Clin Chem 35:1036–1041
Langone JJ, Cook G, Bjercke RJ, Lifschnitz MH (1988) J Immunol Methods 114:73–78
Hennion MC (1999) J Chromatogr A 856:3–54
Sellergren B (1999) Trends Anal Chem 18:164–174
Kandimalla VB, Ju H (2004) Anal Bioanal Chem 380:587–605
Cacho C, Turiel E, Martin-Esteban A, Perez-Conde C, Camara C (2003) Anal Bioanal Chem 376:491–496
Caro E, Marce RM, Cormack PAG, Sherrington DC, Borrull F (2004) J Chromatogr B 813:137–143
Lai JP, Niessner R, Knopp D (2004) Anal Chim Acta 522:137–144
Dong XC, Wang N, Wang SL, Zhang XW, Fan ZJ (2004) J Chromatogr A 1057:13–19
Caro E, Marce RM, Cormack PAG, Sherrington DC, Borrull F (2004) J Chromatogr A 1047:175–180
Deviprasad GR, D’Souza F (2000) Chem Commun 19: 1915–1916
Liu Y, Liu XL, Wang JD (2003) Chin J Anal Chem 31:1202–1206
Wayne MM, Edward PCL, Borje S (1999) Anal Commun 36:217–220
Zander A, Findlay P, Renner T, Sellergren B (1998) Anal Chem 70:3304–3314
Sellergren B (1994) Anal Chem 66:1578–1582
Martin PD, Jones GR, Stringer F, Wilson ID (2003) Analyst 128:345–350
Andrsson LI, Hardenborg E, Sandberg-Stall M, Moller K, Henriksson J, Bramsby-Sjostrom I, Olsson LI, Abdel-Rehim M (2004) Anal Chim Acta 526:147–154
Moller K, Crescenzi C, Nilsson U (2004) Anal Bioanal Chem 378:197–204
Berggren C, Bayoudh S, Sherrington D, Ensing K (2000) J Chromatogr A 889:105–110
Theodoridis G, Manesiotis P (2002) J Chromatogr A 948 163–169
Moller K, Nilsson U, Crescenzi C (2004) J Chromatogr B 811:171–176
Dong XC, Wang W, Ma SJ, Sun H, Li Y, Guo JQ (2005) J Chromatogr A 1070:125–130
Rashid BA, Briggs RJ, Hay JN, Stevenson D (1997) Anal Commun 34:303–305
Bazylak G, Brozik H, Sabanty W (2000) J Pharm Biomed Anal 24:113–123
Spierto FW, Hannon WH, Kendrick JS, Bermert JT, Pirkle J, Gargiullo P (1994) J Smoking Relat Dis 5:65–71
Acknowledgements
This research work has been supported by the National Natural Science Foundation of China (No. 20405013) and the Scientific Foundation of State Tobacco Monopoly Administration of China (No. 110200302026).
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Yang, J., Hu, Y., Cai, JB. et al. A new molecularly imprinted polymer for selective extraction of cotinine from urine samples by solid-phase extraction. Anal Bioanal Chem 384, 761–768 (2006). https://doi.org/10.1007/s00216-005-0221-4
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00216-005-0221-4