Abstract
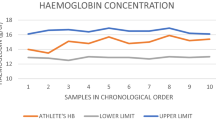
Misuse of recombinant human erythropoietin (rhEPO) is a major concern in competitive sports, and the implementation of tests allowing for higher detection rates than what current tests are capable of is required. In this study, a novel lateral flow EPO isoform test kit, EPO WGA MAIIA, is evaluated on the basis of plasma and urine samples obtained from eight healthy males in connection with a 28-day rhEPO injection period. rhEPO was injected every other day during the first 14 days of the study, and the method proved to be 100 % effective in detecting rhEPO in the concomitantly obtained samples. Seven days after the last injection, three positive (>99.99 % confidence limit (CL)) subjects were found. When using 99 % CL as the cut-off limit, six of the eight subjects (75 %) were found to be suspected of doping. Samples obtained 14 and 21 days after the last injection showed no detectable trace of rhEPO. A previous study using indirect methods to determine EPO doping on the same samples indicated only that two of the subjects had suspicious values 7–21 days after the last injection. We propose implementing the easy to-use EPO WGA MAIIA test as an initial screening procedure in anti-doping work to (1) increase the detection rate of potential rhEPO doping athletes and (2) allow for a 10- to 20-fold higher analytical rate than what is possible today.

The lateral flow isoform test, in a dipstick format, can distinguish different types of EPO (blue balls) due to the interaction of their glycosylated structures with the lectin wheat germ agglutinin (WGA), which is the 1st zone to pass. EPO that have succeeded to pass the WGA zone will be captured by EPO-specific antibodies found in the subsequent zone. The percentage of passed EPO is calculated by also measuring the total amount of EPO in the sample using a dipstick with inactive WGA zone.


Similar content being viewed by others
References
Lundby C, Robach P, Saltin B (2012) The evolving science of detection of 'blood doping'. Br J Pharmacol 165(5):1306–1315
Barroso O, Mazzoni I, Rabin O (2008) Hormone abuse in sports: the antidoping perspective. Asian J Androl 10(3):391–402
Lundby C, Robach P, Boushel R, Thomsen JJ, Rasmussen P, Koskolou M, Calbet JA (2008) Does recombinant human EPO increase exercise capacity by means other than augmenting oxygen transport? J Appl Physiol 105(2):581–587
Halstenson CE, Macres M, Katz SA, Schnieders JR, Watanabe M, Sobota JT, Abraham PA (1991) Comparative pharmacokinetics and pharmacodynamics of epoetin alfa and epoetin beta. Clin Pharmacol Ther 50(6):702–712
Dou P, Liu Z, He J, Xu JJ, Chen HY (2008) Rapid and high-resolution glycoform profiling of recombinant human erythropoietin by capillary isoelectric focusing with whole column imaging detection. J Chromatogr 1190(1–2):372–376
Wang H, Dou P, Lu C, Liu Z (2012) Immuno-magnetic beads-based extraction-capillary zone electrophoresis-deep UV laser-induced fluorescence analysis of erythropoietin. J Chromatogr 1246:48–54
Wide L, Bengtsson C (1990) Molecular charge heterogeneity of human serum erythropoietin. Br J Haematol 76(1):121–127
Wide L, Bengtsson C, Berglund B, Ekblom B (1995) Detection in blood and urine of recombinant erythropoietin administered to healthy men. Med Sci Sports Exerc 27(11):1569–1576
Lasne F, Martin L, Crepin N, de Ceaurriz J (2002) Detection of isoelectric profiles of erythropoietin in urine: differentiation of natural and administered recombinant hormones. Anal Biochem 311(2):119–126
Kohler M, Ayotte C, Desharnais P, Flenker U, Lüdke S, Thevis M, Völker-Schänzer E, Schänzer W (2008) Discrimination of recombinant and endogenous urinary erythropoietin by calculating relative mobility values from SDS gels. Int J Sports Med 29(01):1–6
Reichel C, Kulovics R, Jordan V, Watzinger M, Geisendorfer T (2009) SDS-PAGE of recombinant and endogenous erythropoietins: benefits and limitations of the method for application in doping control. Drug Test Anal 1(1):43–50
Franco Fraguas L, Carlsson J, Lönnberg M (2008) Lectin affinity chromatography as a tool to differentiate endogenous and recombinant erythropoietins. J Chromatogr 1212(1–2):82–88
Lönnberg M, Andren M, Birgegard G, Drevin M, Garle M, Carlsson J (2012) Rapid detection of erythropoiesis-stimulating agents in urine and serum. Anal Biochem 420:101–114
Reichel C (2011) Recent developments in doping testing for erythropoietin. Anal Bioanal Chem 401(2):463–481
Dehnes Y, Lamon S, Lonnberg M (2010) Erythropoietin (EPO) immunoaffinity columns—a powerful tool for purifying EPO and its recombinant analogues. J Pharm Biomed Anal 53(4):1028–1032
Lönnberg M, Dehnes Y, Drevin M, Garle M, Lamon S, Leuenberger N, Quach T, Carlsson J (2010) Rapid affinity purification of erythropoietin from biological samples using disposable monoliths. J Chromatogr 1217(45):7031–7037
Lonnberg M, Bondesson U, Cormant F, Garcia P, Bonnaire Y, Carlsson J, Popot MA, Rollborn N, Rasbo K, Bailly-Chouriberry L (2012) Detection of recombinant human EPO administered to horses using MAIIA lateral flow isoform test. Anal Bioanal Chem 403(6):1619–1628
Ashenden M, Sharpe K, Garnham A, Gore CJ (2012) Evaluation of the MAIIA dipstick test to detect recombinant human erythropoietin in plasma. J Pharm Biomed Anal 67–68:123–128
Lundby C, Achman-Andersen NJ, Thomsen JJ, Norgaard AM, Robach P (2008) Testing for recombinant human erythropoietin in urine: problems associated with current anti-doping testing. J Appl Physiol 105(2):417–419
Lasne F, Martin L, Martin JA, de Ceaurriz J (2007) Isoelectric profiles of human erythropoietin are different in serum and urine. Int J Biol Macromol 41(3):354–357
Lamon S, Robinson N, Sottas P-E, Henry H, Kamber M, Mangin P, Saugy M (2007) Possible origins of undetectable EPO in urine samples. Clin Chim Acta 385(1–2):61–66
Lamon S, Martin L, Robinson N, Saugy M, Ceaurriz J, Lasne F (2009) Effects of exercise on the isoelectric patterns of erythropoietin. Clin J Sport Med 19(4):311–315
Beullens M, Delanghe JR, Bollen M (2006) False-positive detection of recombinant human erythropoietin in urine following strenuous physical exercise. Blood 107(12):4711–4713
Jelkmann W (2007) Novel erythropoietic agents: a threat to sportsmanship. Medicina Sportiva 11(2):32–42
Lönnberg M, Drevin M, Carlsson J (2008) Ultra-sensitive immunochromatographic assay for quantitative determination of erythropoietin. J Immunol Methods 339(2):236–244
Zorzoli M (2005) Blood monitoring in anti-doping setting. Recent Adv Doping Anal 13:255–264
Sharpe K, Ashenden MJ, Schumacher YO (2006) A third generation approach to detect erythropoietin abuse in athletes. Haematologica 91(3):356–363
Borno A, Aachmann-Andersen NJ, Munch-Andersen T, Hulston CJ, Lundby C (2010) Screening for recombinant human erythropoietin using [Hb], reticulocytes, the OFF(hr score), OFF(z score) and Hb(z score): status of the Blood Passport. Eur J Appl Physiol 109(3):537–543
Franz SE (2009) Erythropoiesis-stimulating agents: development, detection and dangers. Drug Test Anal 1(6):245–249
Macdougall IC, Ashenden M (2009) Current and upcoming erythropoiesis-stimulating agents, iron products, and other novel anemia medications. Adv Chronic Kidney Dis 16(2):117–130
Dehnes Y, Hemmersbach P (2011) Effect of single doses of methoxypolyethylene glycol-epoetin beta (CERA, Mircera) and epoetin delta (Dynepo) on isoelectric erythropoietin profiles and haematological parameters. Drug Test Anal 3(5):291–299
Acknowledgments
The authors thank Maria Andrén, Niklas Rollborn, Malin Drevin, Mikael Lönnberg and Trikien Quach for technical assistance. The authors are grateful for the financial support for the development of the MAIIA test from VINNOVA (The Swedish Governmental Agency for Innovation Systems) in the project P25917.
Author information
Authors and Affiliations
Corresponding author
Additional information
Published in the topical collection Anti-doping Analysis with guest editor Christopher Harrison.
Rights and permissions
About this article
Cite this article
Lönnberg, M., Lundby, C. Detection of EPO injections using a rapid lateral flow isoform test. Anal Bioanal Chem 405, 9685–9691 (2013). https://doi.org/10.1007/s00216-013-6997-8
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00216-013-6997-8