Abstract
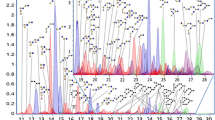
Human milk oligosaccharides (HMO) are one of the major components of human milk. HMO are non-digestible by the human gut, where they are known to play important functions as prebiotics and decoys for binding pathogens. Moreover, it has been proposed that HMO may provide sialic acids to the infant that are important in brain development, however this would require absorption of HMO into the bloodstream. HMO have consistently been found in the urine of humans and other mammals, suggesting systemic absorption. Here, we present a procedure for the profiling of milk oligosaccharides (MO) in plasma samples obtained from 13 term infants hospitalized for surgery for congenital heart disease. The method comprises protein denaturation, oligosaccharide reduction, and porous graphitized carbon solid phase extraction for purification followed by analysis using nHPLC-PGC-chip-TOF-MS. Approximately 15 free MO were typically observed in the plasma of human infants, including LNT, LDFP, LNFT, 3′SL, 6′SL, 3′SLN, and 6′SLN, of which the presence was confirmed using fragmentation studies. A novel third isomer of SLN, not found in human or bovine milk was also consistently detected. Differences in the free MO profiles were observed between infants that were totally formula-fed and infants that received at least some part breast milk. Our results indicate that free MO similar in structure to those found in human milk and urine are present in the blood of infants. The method and results presented here will facilitate further research toward the possible roles of free MO in the development of the infant.





Similar content being viewed by others
Abbreviations
- FA:
-
Formic acid
- FL:
-
Fucosyllactose
- Fuc:
-
Fucose
- Gal:
-
Galactose
- Glc:
-
Glucose
- GlcNAc:
-
N-acetylglucosamine
- HMO:
-
Human milk oligosaccharide
- LDFP:
-
Lacto-difucosyl-pentaose
- LNFP:
-
Lacto-N-fucopentaose
- LNnT:
-
Lacto-N-neotetraose
- LNT:
-
Lacto-N-tetraose
- MO:
-
Milk oligosaccharide
- Neu5Ac:
-
N-acetylneuraminic acid
- PGC:
-
Porous graphitized carbon
- RSD:
-
Relative standard deviation
- SL:
-
Sialyllactose
- SLN:
-
Sialyllactosamine
- TFA:
-
Trifluoroacetic acid
References
German JB, Freeman SL, Lebrilla CB, Mills DA (2008) Human milk oligosaccharides: evolution, structures and bioselectivity as substrates for intestinal bacteria. Nestle Nutr Workshop Ser Pediatr Program 62:205–218. doi:10.1159/000146322, discussion 218–222
Zivkovic AM, German JB, Lebrilla CB, Mills DA (2011) Human milk glycobiome and its impact on the infant gastrointestinal microbiota. Proc Natl Acad Sci U S A 108(1):4653–4658. doi:10.1073/pnas.1000083107
Coppa GV, Bruni S, Morelli L, Soldi S, Gabrielli O (2004) The first prebiotics in humans: human milk oligosaccharides. J Clin Gastroenterol 38(6 Suppl):S80–S83
Marcobal A, Sonnenburg JL (2012) Human milk oligosaccharide consumption by intestinal microbiota. Clin Microbiol Infect 18(Suppl 4):12–15. doi:10.1111/j.1469-0691.2012.03863.x
Guaraldi F, Salvatori G (2012) Effect of breast and formula feeding on gut microbiota shaping in newborns. Front Cell Infect Microbiol 2:94. doi:10.3389/fcimb.2012.00094
Newburg DS (2005) Innate immunity and human milk. J Nutr 135(5):1308–1312
Stepans MB, Wilhelm SL, Hertzog M, Rodehorst TK, Blaney S, Clemens B, Polak JJ, Newburg DS (2006) Early consumption of human milk oligosaccharides is inversely related to subsequent risk of respiratory and enteric disease in infants. Breastfeed Med 1(4):207–215. doi:10.1089/bfm.2006.1.207
Wang B (2012) Molecular mechanism underlying sialic acid as an essential nutrient for brain development and cognition. Adv Nutr 3(3):465S–472S. doi:10.3945/An.112.001875
Section on B (2012) Breastfeeding and the use of human milk. Pediatrics 129 (3):e827-841. doi:10.1542/peds.2011-3552
Meinzen-Derr J, Poindexter B, Wrage L, Morrow AL, Stoll B, Donovan EF (2009) Role of human milk in extremely low birth weight infants’ risk of necrotizing enterocolitis or death. J Perinatol 29(1):57–62. doi:10.1038/jp.2008.117
Mai V, Young CM, Ukhanova M, Wang X, Sun Y, Casella G, Theriaque D, Li N, Sharma R, Hudak M, Neu J (2011) Fecal microbiota in premature infants prior to necrotizing enterocolitis. PLoS ONE 6(6):e20647. doi:10.1371/journal.pone.0020647
Tao N, Wu S, Kim J, An HJ, Hinde K, Power ML, Gagneux P, German JB, Lebrilla CB (2011) Evolutionary glycomics: characterization of milk oligosaccharides in primates. J Proteome Res 10(4):1548–1557. doi:10.1021/pr1009367
Wu S, Grimm R, German JB, Lebrilla CB (2011) Annotation and structural analysis of sialylated human milk oligosaccharides. J Proteome Res 10(2):856–868. doi:10.1021/pr101006u
Wu S, Tao N, German JB, Grimm R, Lebrilla CB (2010) Development of an annotated library of neutral human milk oligosaccharides. J Proteome Res 9(8):4138–4151. doi:10.1021/pr100362f
Aldredge DL, Geronimo MR, Hua S, Nwosu CC, Lebrilla CB, Barile D (2013) Annotation and structural elucidation of bovine milk oligosaccharides and determination of novel fucosylated structures. Glycobiology 23(6):664–676. doi:10.1093/glycob/cwt007
Rudloff S, Pohlentz G, Borsch C, Lentze MJ, Kunz C (2012) Urinary excretion of in vivo (1)(3)C-labelled milk oligosaccharides in breastfed infants. Br J Nutr 107(7):957–963. doi:10.1017/S0007114511004016
Rudloff S, Pohlentz G, Diekmann L, Egge H, Kunz C (1996) Urinary excretion of lactose and oligosaccharides in preterm infants fed human milk or infant formula. Acta Paediatr 85(5):598–603
Obermeier S, Rudloff S, Pohlentz G, Lentze MJ, Kunz C (1999) Secretion of 13C-labelled oligosaccharides into human milk and infant's urine after an oral [13C]galactose load. Isotopes Environ Health Stud 35(1–2):119–125. doi:10.1080/10256019908234084
Chaturvedi P, Warren CD, Buescher CR, Pickering LK, Newburg DS (2001) Survival of human milk oligosaccharides in the intestine of infants. Adv Exp Med Biol 501:315–323
Santos-Fandila A, Zafra-Gómez A, Vazquez E, Navalón A, Rueda R, Ramírez M (2013) Ultra high performance liquid chromatography–tandem mass spectrometry method for the determination of soluble milk glycans in rat serum. Talanta doi: 10.1016/j.talanta.2013.10.013
Ruhaak LR, Deelder AM, Wuhrer M (2009) Oligosaccharide analysis by graphitized carbon liquid chromatography-mass spectrometry. Anal Bioanal Chem 394(1):163–174. doi:10.1007/s00216-009-2664-5
Ninonuevo M, An H, Yin H, Killeen K, Grimm R, Ward R, German B, Lebrilla C (2005) Nanoliquid chromatography-mass spectrometry of oligosaccharides employing graphitized carbon chromatography on microchip with a high-accuracy mass analyzer. Electrophoresis 26(19):3641–3649
De Leoz ML, Wu S, Strum JS, Ninonuevo MR, Gaerlan SC, Mirmiran M, German JB, Mills DA, Lebrilla CB, Underwood MA (2013) A quantitative and comprehensive method to analyze human milk oligosaccharide structures in the urine and feces of infants. Anal Bioanal Chem 405(12):4089–4105. doi:10.1007/s00216-013-6817-1
Totten SM, Zivkovic AM, Wu S, Ngyuen U, Freeman SL, Ruhaak LR, Darboe MK, German JB, Prentice AM, Lebrilla CB (2012) Comprehensive profiles of human milk oligosaccharides yield highly sensitive and specific markers for determining secretor status in lactating mothers. J Proteome Res 11(12):6124–6133. doi:10.1021/pr300769g
Ninonuevo MR, Park Y, Yin H, Zhang J, Ward RE, Clowers BH, German JB, Freeman SL, Killeen K, Grimm R, Lebrilla CB (2006) A strategy for annotating the human milk glycome. J Agric Food Chem 54(20):7471–7480
Ellis CL, Bokulich NA, Kalanetra KM, Mirmiran M, Elumalai J, Haapanen L, Schegg T, Rutledge JC, Raff G, Mills DA, Underwood MA (2013) Probiotic administration in congenital heart disease: a pilot study. J Perinatol 33(9):691–697. doi:10.1038/jp.2013.41
Blank D, Gebhardt S, Maass K, Lochnit G, Dotz V, Blank J, Geyer R, Kunz C (2011) High-throughput mass finger printing and Lewis blood group assignment of human milk oligosaccharides. Anal Bioanal Chem 401(8):2495–2510. doi:10.1007/s00216-011-5349-9
Thurl S, Munzert M, Henker J, Boehm G, Muller-Werner B, Jelinek J, Stahl B (2010) Variation of human milk oligosaccharides in relation to milk groups and lactational periods. Br J Nutr 104(9):1261–1271. doi:10.1017/S0007114510002072
Tao N, DePeters EJ, Freeman S, German JB, Grimm R, Lebrilla CB (2008) Bovine milk glycome. J Dairy Sci 91(10):3768–3778. doi:10.3168/jds.2008-1305
Kim JH, An HJ, Garrido D, German JB, Lebrilla CB, Mills DA (2013) Proteomic analysis of Bifidobacterium longum subsp. infantis reveals the metabolic insight on consumption of prebiotics and host glycans. PLoS ONE 8(2):e57535
Ruhaak LR, Taylor SL, Miyamoto S, Kelly K, Leiserowitz GS, Gandara D, Lebrilla CB, Kim K (2013) Chip-based nLC-TOF-MS is a highly stable technology for large-scale high-throughput analyses. Anal Bioanal Chem 405(14):4953–4958. doi:10.1007/s00216-013-6908-z
Acknowledgments
Funding was provided by the NIH (R01 HD059127 and UL1 TR000002); the authors declare no conflict of interest.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Ruhaak, L.R., Stroble, C., Underwood, M.A. et al. Detection of milk oligosaccharides in plasma of infants. Anal Bioanal Chem 406, 5775–5784 (2014). https://doi.org/10.1007/s00216-014-8025-z
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00216-014-8025-z