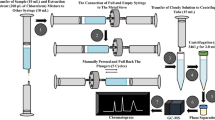
Abstract
Among the new psychoactive substances (NPS), so-called designer benzodiazepines have become of particular importance over the last 2 years, due to their increasing availability on the internet drug market. Therapeutically used nitrobenzodiazepines such as flunitrazepam are known to be extensively metabolized via N-dealkylation to active metabolites and via nitro reduction to the 7-amino compounds. The aim of the present work was to tentatively identify phase I and II metabolites of the latest members of this class appearing on the NPS market, clonazolam, meclonazepam, and nifoxipam, in human urine samples. Nano-liquid chromatography-high-resolution mass spectrometry was used to provide data about their detectability in urine. Data revealed that clonazolam and meclonazepam were extensively metabolized and mainly excreted as their amino and acetamino metabolites. Nifoxipam was also extensively metabolized, but instead mainly excreted as the acetamino metabolite and a glucuronic acid conjugate of the parent. Based on analysis of human urine samples collected in cases of acute intoxication within the Swedish STRIDA project, and samples submitted for routine drug testing, the most abundant metabolites and good targets for urine drug testing were 7-aminoclonazolam for clonazolam, 7-acetaminomeclonazepam for meclonazepam, and 7-acetaminonifoxipam for nifoxipam.








Similar content being viewed by others
References
UNODC. World Drug Report United Nations publication Sales No. E.14.XI.7. 2014.
EMCDDA. New psychoactive substances in Europe. EMCDDA Annual Report, 2015.
UNODC. World Drug Report United Nations publication Sales No. E.15.XI.6, 2015.
Schifano F, Orsolini L, Duccio Papanti G, Corkery JM. Novel psychoactive substances of interest for psychiatry. World Psychiatry. 2015;14(1):15–26.
Helander A, Beck O, Backberg M. Intoxications by the dissociative new psychoactive substances diphenidine and methoxphenidine. Clin Toxicol. 2015;53(5):446–53.
Helander A, Backberg M, Beck O. MT-45, a new psychoactive substance associated with hearing loss and unconsciousness. Clin Toxicol. 2014;52(8):901–4.
Backberg M, Lindeman E, Beck O, Helander A. Characteristics of analytically confirmed 3-MMC-related intoxications from the Swedish STRIDA project. Clin Toxicol. 2015;53(1):46–53.
Backberg M, Beck O, Jonsson KH, Helander A. Opioid intoxications involving butyrfentanyl, 4-fluorobutyrfentanyl, and fentanyl from the Swedish STRIDA project. Clin Toxicol. 2015;53(7):609–17.
Backberg M, Beck O, Hulten P, Rosengren-Holmberg J, Helander A. Intoxications of the new psychoactive substance 5-(2-aminopropyl)indole (5-IT): a case series from the Swedish STRIDA project. Clin Toxicol. 2014;52(6):618–24.
Moosmann B, King LA, Auwarter V. Designer benzodiazepines: a new challenge. World Psychiatry. 2015;14(2):248.
Moosmann B, Hutter M, Huppertz LM, Ferlaino S, Redlingshofer L, Auwarter V. Characterization of the designer benzodiazepine pyrazolam and its detectability in human serum and urine. Forensic Toxicol. 2013;31(2):263–71.
Moosmann B, Huppertz LM, Hutter M, Buchwald A, Ferlaino S, Auwarter V. Detection and identification of the designer benzodiazepine flubromazepam and preliminary data on its metabolism and pharmacokinetics. J Mass Spectrom. 2013;48(11):1150–9.
Moosmann B, Bisel P, Auwarter V. Characterization of the designer benzodiazepine diclazepam and preliminary data on its metabolism and pharmacokinetics. Drug Test Anal. 2014;6(7–8):757–63.
Helander A, Beck O, Hagerkvist R, Hulten P. Identification of novel psychoactive drug use in Sweden based on laboratory analysis—initial experiences from the STRIDA project. Scand J Clin Lab Invest. 2013;73(5):400–6.
Helander A, Backberg M, Hulten P, Al-Saffar Y, Beck O. Detection of new psychoactive substance use among emergency room patients: results from the Swedish STRIDA project. Forensic Sci Int. 2014;243:23–9.
Danneberg P, Weber KH. Chemical structure and biological activity of the diazepines. Br J Clin Pharmacol. 1983;16 Suppl 2:231S–44S.
Menezes CM, Rivera G, Alves MA, do Amaral DN, Thibaut JP, Noel F, et al. Synthesis, biological evaluation, and structure-activity relationship of clonazepam, meclonazepam, and 1,4-benzodiazepine compounds with schistosomicidal activity. Chem Biol Drug Des. 2012;79(6):943–9.
Mahajan A, Kumar V, Mansour NR, Bickle Q, Chibale K. Meclonazepam analogues as potential new antihelmintic agents. Bioorg Med Chem Lett. 2008;18(7):2333–6.
elSohly MA, Feng S, Salamone SJ, Wu R. A sensitive GC-MS procedure for the analysis of flunitrazepam and its metabolites in urine. J Anal Toxicol. 1997;21(5):335–40.
Hoiseth G, Middelkoop G, Morland J, Gjerde H. Has previous abuse of flunitrazepam been replaced by clonazepam? Eur Addict Res. 2015;21(4):217–21.
Druid H, Holmgren P, Ahlner J. Flunitrazepam: an evaluation of use, abuse and toxicity. Forensic Sci Int. 2001;122(2–3):136–41.
Jones JD, Mogali S, Comer SD. Polydrug abuse: a review of opioid and benzodiazepine combination use. Drug Alcohol Depend. 2012;125(1–2):8–18.
LinWu SW, Wu CA, Peng FC, Wang AH. Structure-based development of bacterial nitroreductase against nitrobenzodiazepine-induced hypnosis. Biochem Pharmacol. 2012;83(12):1690–9.
Greenblatt DJ, Shader RI, Divoll M, Harmatz JS. Benzodiazepines: a summary of pharmacokinetic properties. Br J Clin Pharmacol. 1981;11 Suppl 1:11S–6S.
Breimer DD. Pharmacokinetics and metabolism of various benzodiazepines used as hypnotics. Br J Clin Pharmacol. 1979;8(1):7S–13S.
Huppertz LM, Bisel P, Westphal F, Franz F, Auwarter V, Moosmann B. Characterization of the four designer benzodiazepines clonazolam, deschloroetizolam, flubromazolam, and meclonazepam, and identification of their in vitro metabolites. Forensic Toxicol. 2015;33(2):388–95.
Spaggiari D, Geiser L, Rudaz S. Coupling ultra-high-pressure liquid chromatography with mass spectrometry for in-vitro drug-metabolism studies. Trac Trend Anal Chem. 2014;63:129–39.
Rainville PD, Smith NW, Wilson ID, Nicholson JK, Plumb RS. Addressing the challenge of limited sample volumes in in vitro studies with capillary-scale microfluidic LC-MS/MS. Bioanalysis. 2011;3(8):873–82.
Valaskovic GA, Utley L, Lee MS, Wu JT. Ultra-low flow nanospray for the normalization of conventional liquid chromatography/mass spectrometry through equimolar response: standard-free quantitative estimation of metabolite levels in drug discovery. Rapid Commun Mass Spectrom. 2006;20(7):1087–96.
Wickremsinhe ER, Singh G, Ackermann BL, Gillespie TA, Chaudhary AK. A review of nanoelectrospray ionization applications for drug metabolism and pharmacokinetics. Curr Drug Metab. 2006;7(8):913–28.
Schadt S, Chen LZ, Bischoff D. Evaluation of relative LC/MS response of metabolites to parent drug in LC/nanospray ionization mass spectrometry: potential implications in MIST assessment. J Mass Spectrom. 2011;46(12):1281–6.
Forsman M, Nystrom I, Roman M, Berglund L, Ahlner J, Kronstrand R. Urinary detection times and excretion patterns of flunitrazepam and its metabolites after a single oral dose. J Anal Toxicol. 2009;33(8):491–501.
Negrusz A, Moore CM, Stockham TL, Poiser KR, Kern JL, Palaparthy R, et al. Elimination of 7-aminoflunitrazepam and flunitrazepam in urine after a single dose of Rohypnol. J Forensic Sci. 2000;45(5):1031–40.
Coassolo P, Aubert C, Cano JP. Plasma determination of 3-methylclonazepam by capillary gas chromatography. J Chromatogr. 1985;338(2):347–55.
Niessen WM. Fragmentation of toxicologically relevant drugs in positive-ion liquid chromatography-tandem mass spectrometry. Mass Spectrom Rev. 2011;30(4):626–63.
Smyth WF, McClean S, Ramachandran VN. A study of the electrospray ionisation of pharmacologically significant 1,4-benzodiazepines and their subsequent fragmentation using an ion-trap mass spectrometer. Rapid Commun Mass Spectrom. 2000;14(21):2061–9.
Smyth WF, Joyce C, Ramachandran VN, O’ Kane E, Coulter D. Characterisation of selected hypnotic drugs and their metabolites using electrospray ionisation with ion trap mass spectrometry and with quadrupole time-of-flight mass spectrometry and their determination by liquid chromatography-electrospray ionisation-ion trap mass spectrometry. Anal Chim Acta. 2004;506(2):203–14.
Bourcier K, Hyland R, Kempshall S, Jones R, Maximilien J, Irvine N, et al. Investigation into UDP-glucuronosyltransferase (UGT) enzyme kinetics of imidazole- and triazole-containing antifungal drugs in human liver microsomes and recombinant UGT enzymes. Drug Metab Dispos. 2010;38(6):923–9.
Court MH, Hao Q, Krishnaswamy S, Bekaii-Saab T, Al-Rohaimi A, von Moltke LL, et al. UDP-glucuronosyltransferase (UGT) 2B15 pharmacogenetics: UGT2B15 D85Y genotype and gender are major determinants of oxazepam glucuronidation by human liver. J Pharmacol Exp Ther. 2004;310(2):656–65.
Meyer MR, Robert A, Maurer HH. Toxicokinetics of novel psychoactive substances: characterization of N-acetyltransferase (NAT) isoenzymes involved in the phase II metabolism of 2C designer drugs. Toxicol Lett. 2014;227(2):124–8.
Schwaninger AE, Meyer MR, Zapp J, Maurer HH. The role of human UDP-glucuronyltransferases on the formation of the methylenedioxymethamphetamine (ecstasy) phase II metabolites R- and S-3-methoxymethamphetamine 4-O-glucuronides. Drug Metab Dispos. 2009;37(11):2212–20.
Acknowledgments
The STRIDA project was supported by grants from the Public Health Agency of Sweden.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
The study was conducted in accordance with the Helsinki Declaration. The use of de-identified leftover volumes from the routine samples pool (No. 00-230) and the STRIDA project (No. 2013/116–31/2) were approved by the regional ethical review board.
Conflict of interest
The authors declare that they have no conflicts of interest.
Electronic supplementary material
Below is the link to the electronic supplementary material.
ESM 1
(PDF 112 kb)
Rights and permissions
About this article
Cite this article
Meyer, M.R., Bergstrand, M.P., Helander, A. et al. Identification of main human urinary metabolites of the designer nitrobenzodiazepines clonazolam, meclonazepam, and nifoxipam by nano-liquid chromatography-high-resolution mass spectrometry for drug testing purposes. Anal Bioanal Chem 408, 3571–3591 (2016). https://doi.org/10.1007/s00216-016-9439-6
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00216-016-9439-6