Abstract
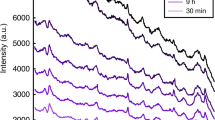
Knowing the time since deposition (TSD) of an evidentiary bloodstain is highly desired in forensics, yet it can be extremely complicated to accurately determine in practice. Although there have been numerous attempts to solve this problem using a variety of different techniques, currently, no established, well-accepted method exists. Here, a Raman spectroscopic approach was developed for determining the age of bloodstains up to 1 week old. Raman spectroscopy, along with two-dimensional correlation spectroscopy (2D CoS) and statistical modeling, was used to analyze fresh bloodstains at ten time points under ambient conditions. The 2D CoS results indicate a high correlation between several Raman bands and the age of a bloodstain. A regression model was built to provide quantitative predictions of the TSD, with cross-validated root mean squared error and R 2 values of 0.13 and 0.97, respectively. It was determined that a “new” (1 h) bloodstain could be easily distinguished from older bloodstains, which is very important for forensic science in helping to establish the relevant association of multiple bloodstains. Additionally, all bloodstains were confirmatively identified as blood by comparing the experimentally measured spectra to multidimensional body fluid spectroscopic signatures of blood, saliva, semen, sweat, and vaginal fluid. These results demonstrate that Raman spectroscopy can be used as a nondestructive analytical tool for discriminating between bloodstains on the scale of hours to days. This approach shows promise for immediate practical use in the field to predict the TSD with a high degree of accuracy.

Bloodstain aging over time illustrating naturally ocurring processes






Similar content being viewed by others
References
Lee HC, Palmbach T, Miller MT. Crime scene reconstruction. Henry Lee’s crime scene handbook. San Diego, CA: Elsevier Academic Press; 2001. p. 271–98.
Castro DM, Coyle HM. Review: biological evidence collection and forensic blood identification. West Haven, CT: Identacode Consulting LLC; 2013.
James SH, Kish PE, Sutton TP. Principles of bloodstain pattern analysis: theory and practice. Boca Raton, FL: CRC Press; 2005
Bevel T, Gardner RM. Bloodstain pattern analysis with an introduction to crime scene reconstruction. Boca Raton, FL: CRC Press; 2008
Bremmer RH, de Bruin KG, van Gemert MJC, van Leeuwen TG, Aalders MCG. Forensic quest for age determination of bloodstains. Forensic Sci Int. 2012;216(1–3):1–11.
Spalding RP. Identification and characterization of blood and bloodstains. In: James SH, Nordby JJ, Bell S, editors. Forensic science: an introduction to scientific and investigative techniques. Boca Raton: CRC Press; 2009. p. 181–201.
Kobilinsky L. Forensic chemistry handbook. Hoboken, NJ: John Wiley & Sons; 2012.
Vandenberg N, van Oorshot RAH. The use of Polilight in the detection of seminal fluid, saliva, and bloodstains and comparison with conventional chemical-based screening tests. J Forensic Sci. 2006;51(2):361–70.
Webb JL, Creamer JI, Quickenden TI. A comparison of the presumptive luminol test for blood with four non-chemiluminescent forensic techniques. Luminescence. 2006;21:214–20.
Lin AC-Y, Hsieh H-M, Tsai L-C, Linacre A, Lee JC-I. Forensic applications of infrared imaging for the detection and recording of latent evidence. J Forensic Sci. 2007;52(5):1148–50.
Johnston E, Ames CE, Dagnall KE, Foster J, Daniel BE. Comparison of presumptive blood test kits including Hexagon OBTI. J Forensic Sci. 2008;53(3):687–9.
Brooke H, Baranowski MR, McCutcheon JN, Morgan SL, Myrick ML. Multimode imaging in the thermal infrared for chemical contrast enhancement. Part 3: visualizing blood on fabrics. Anal Chem. 2010;82(20):8427–31.
Dixon TR, Samudra AV, Stewart Jr WD, Johari O. A scanning electron microscope study of dried blood. J Forensic Sci. 1976;21(4):797–803.
Sottolano S, de Forest PR. An improved technique for the preparation of Teichman and Takayama crystals from blood. The Microscope. 1980;28(2):41–6.
Kotowski TM, Grieve MC. The use of microspectrophotometry to characterize microscopic amounts of blood. J Forensic Sci. 1986;31(3):1079–85.
Kashyap VK. A simple immunosorbent assay for detection of human blood. J Immunoassay. 1989;10(4):315–24.
Ballantyne J, Juusola J, inventors; Research Foundation of The University of Central Florida, Inc., assignee. Messenger RNA profiling: body fluid identification using multiplex reverse transcription-polymerase chain reaction (RT-PCR). USA 2007.
Bauer M. RNA in forensic science. Forensic Sci Int Genet. 2007;1:69–74.
Bauer M, Patzelt D. Identification of menstrual blood by real time RT-PCR: technical improvements and the practical value of negative test results. Forensic Sci Int. 2008;174:55–9.
Haas C, Klesser B, Maake C, Bär W, Kratzer A. mRNA profiling for body fluid identification by reverse transcription endpoint PCR and realtime PCR. Forensic Sci Int Genet. 2009;3(2):80–8.
Virkler K, Lednev IK. Analysis of body fluids for forensic purposes: from laboratory testing to non-destructive rapid confirmatory identification at a crime scene. Forensic Sci Int. 2009;188(1–3):1–17.
Sikirzhytski V, Sikirzhytskaya A, Lednev IK. Multidimensional Raman spectroscopic signatures as a tool for forensic identification of body fluid traces: a review. Appl Spectrosc. 2011;65(11):1223–32.
Virkler K, Lednev IK. Raman spectroscopic signature of blood and its potential application to forensic body fluid identification. Anal Bioanal Chem. 2010;396(1):525–34.
McLaughlin G, Lednev IK. A modified Raman multidimensional spectroscopic signature of blood to account for the effect of laser power. Forensic Sci Int. 2014;240:88–94.
Virkler K, Lednev IK. Forensic body fluid identification: the Raman spectroscopic signature of saliva. Analyst. 2010;135(3):512–7.
Virkler K, Lednev IK. Raman spectroscopic signature of semen and its potential application to forensic body fluid identification. Forensic Sci Int. 2009;193(1–3):56–62.
Sikirzhytski V, Sikirzhytskaya A, Lednev IK. Multidimensional Raman spectroscopic signature of sweat and its potential application to forensic body fluid identification. Anal Chim Acta. 2012;718:78–83.
Sikirzhytskaya A, Sikirzhytski V, Lednev IK. Raman spectroscopic signature of vaginal fluid and its potential application in forensic body fluid identification. Forensic Sci Int. 2012;216(1–3):44–8.
Virkler K, Lednev IK. Blood species identification for forensic purposes using Raman spectroscopy combined with advanced statistical analysis. Anal Chem. 2009;81(18):7773–7.
McLaughlin G, Doty KC, Lednev IK. Discrimination of human and animal blood traces via Raman spectroscopy. Forensic Sci Int. 2014;238:91–5.
McLaughlin G, Doty KC, Lednev IK. Raman spectroscopy of blood for species identification. Anal Chem. 2014;86(23):11628–33.
Sikirzhytskaya A, Sikirzhytski V, Lednev IK. Raman spectroscopy coupled with advanced statistics for differentiating menstrual and peripheral blood. J Biophotonics. 2014;7(1–2):59–67.
McLaughlin G, Sikirzhytski V, Lednev IK. Circumventing substrate interference in the Raman spectroscopic identification of blood stains. Forensic Sci Int. 2013;231(1–3):157–66.
McLaughlin G, Lednev IK. In situ identification of semen stains on common substrates via Raman spectroscopy. J Forensic Sci. 2015;595–604.
Sikirzhytskaya A, Sikirzhytski V, McLaughlin G, Lednev IK. Forensic identification of blood in the presence of contaminations using Raman microspectroscopy coupled with advanced statistics: effect of sand, dust, and soil. J Forensic Sci. 2013;58(5):1141–8.
Sikirzhytski V, Sikirzhytskaya A, Lednev IK. Advanced statistical analysis of Raman spectroscopic data for the identification of body fluid traces: semen and blood mixtures. Forensic Sci Int. 2012;222(1–3):259–65.
Marrone A, Ballantyne J. Changes in dry state hemoglobin over time do not increase the potential for oxidative DNA damage in dried blood. PLoS One. 2009;4(4):e5110-e-8.
Asghari-Khiavi M, Mechler A, Bambery KR, McNaughton D, Wood BR. A resonance Raman spectroscopic investigation into the effects of fixation and dehydration on heme environment of hemoglobin. J Raman Spectrosc. 2009;40(11):1668–74.
Anderson S, Howard B, Hobbs GR, Bishop CP. A method for determining the age of a bloodstain. Forensic Sci Int. 2005;148(1):37–45.
Alrowaithi MA, McCallum NA, Watson ND. A method for determining the age of a bloodstain. Forensic Sci Int. 2014;234:e30–1.
Bauer M, Polzin S, Patzelt D. Quantification of RNA degradation by semi-quantitative duplex and competitive RT-PCR: a possible indicator of the age of bloodstains? Forensic Sci Int. 2003;138(1–3):94–103.
Andrasko J. The estimation of age of bloodstains by HPLC analysis. J Forensic Sci. 1997;42(4):601–7.
Inoue H, Takabe F, Iwasa M, Maeno Y. Identification of fetal hemoglobin and simultaneous estimation of bloodstain age by high-performance liquid chromatography. Int J Legal Med. 1991;104(3):127–31.
Bremmer RH, Nadort A, van Leeuwen TG, van Gemert MJC, Aalders MCG. Age estimation of blood stains by hemoglobin derivative determination using reflectance spectroscopy. Forensic Sci Int. 2011;206(1–3):166–71.
Bremmer RH, Edelman G, Vegter TD, Bijvoets T, Aalders MCG. Remote spectroscopic identification of bloodstains. J Forensic Sci. 2011;56(6):1471–5.
Strasser S, Zink A, Kada G, Hinterdorfer P, Peschel O, Heckl WM, et al. Age determination of blood spots in forensic medicine by force spectroscopy. Forensic Sci Int. 2007;170(1):8–14.
Hanson EK, Ballantyne J. A blue spectral shift of the hemoglobin soret band correlates with the age (time since deposition) of dried bloodstains. PLoS One. 2010;5(9):e12830-1–e12830-11.
Fujita Y, Tsuchiya K, Abe S, Takiguchi Y, Kubo S, Sakurai H. Estimation of the age of human bloodstains by electron paramagnetic resonance spectroscopy: long-term controlled experiment on the effects of environmental factors. Forensic Sci Int. 2005;152(1):39–43.
Botonjic-Sehic E, Brown CW, Lamontagne M, Tsaparikos M. Forensic application of near-infrared spectroscopy: aging of bloodstains. Spectroscopy. 2009;24(2):42–8.
Dasgupta R, Ahlawat S, Verma RS, Uppal A, Gupta PK. Hemoglobin degradation in human erythrocytes with long-duration near-infrared laser exposure in Raman optical tweezers. J Biomed Opt. 2010;15(5):055009–11.
Boyd S, Bertino MF, Seashols SJ. Raman spectroscopy of blood samples for forensic applications. Forensic Sci Int. 2011;208(1–3):124–8.
Thanakiatkrai P, Yaodam A, Kitpipit T. Age estimation of bloodstains using smartphones and digital image analysis. Forensic Sci Int. 2013;233(1–3):288–97.
Agudelo J, Huynh C, Halamek J. Forensic determination of blood sample age using a bioaffinity-based assay. Analyst. 2015;140(5):1411–5.
Guo K, Achilefu S, Berezin MY. Dating bloodstains with fluorescence lifetime measurements. Chem Eur J. 2012;18(5):1303–5.
Li B, Beveridge P, O’Hare WT, Islam M. The estimation of the age of a blood stain using reflectance spectroscopy with a microspectrophotometer, spectral pre-processing and linear discriminant analysis. Forensic Sci Int. 2011;212(1–3):198–204.
Li B, Beveridge P, O’Hare WT, Islam M. The age estimation of blood stains up to 30 days old using visible wavelength hyperspectral image analysis and linear discriminant analysis. Sci Justice. 2013;53(3):270–7.
Edelman G, van Leeuwen TG, Aalders MCG. Hyperspectral imaging for the age estimation of blood stains at the crime scene. Forensic Sci Int. 2012;223(1–3):72–7.
Edelman GJ, Gaston E, van Leeuwen TG, Cullen PJ, Aalders MCG. Hyperspectral imaging for non-contact analysis of forensic traces. Forensic Sci Int. 2012;223(1–3):28–39.
Premasiri WR, Lee JC, Ziegler LD. Surface-enhanced Raman scattering of whole human blood, blood plasma, and red blood cells: cellular processes and bioanalytical sensing. J Phys Chem B. 2012;116(31):9376–86.
Lemler P, Premasiri WR, DelMonaco A, Ziegler LD. NIR Raman spectra of whole human blood: effects of laser-induced and in vitro hemoglobin denaturation. Anal Bioanal Chem. 2014;406(1):193–200.
Noda I, Ozaki Y. Two-dimensional correlation spectroscopy: applications in vibrational and optical spectroscopy. Hoboken, NJ: Wiley; 2004.
Cui L, Butler HJ, Martin-Hirsch PL, Martin FL. Aluminium foil as a potential substrate for ATR-FTIR, transflection FTIR or Raman spectrochemical analysis of biological specimens. Anal Methods. 2016;8(3):481–7.
Wood BR, Hammer L, Davis L, McNaughton D. Raman microspectroscopy and imaging provides insights into heme aggregation and denaturation within human erythrocytes. J Biomed Opt. 2005;10(1):014005–01400513.
Rygula A, Majzner K, Marzec KM, Kaczor A, Pilarczyk M, Baranska M. Raman spectroscopy of proteins: a review. J Raman Spectrosc. 2013;44(8):1061–76.
Bruin J. FAQ: what is the coefficient of variation? UCLA: Statistical Consulting Group; 2011. Available from: http://www.ats.ucla.edu/stat/mult_pkg/faq/general/coefficient_of_variation.htm.
Acknowledgments
This project was supported by Awards No. 2011-DN-BX-K551, 2014-DN-BX-K016, and 2015-R2-CX-0021 awarded by the National Institute of Justice, Office of Justice Programs, U.S. Department of Justice (I.K.L.). The opinions, findings, and conclusions or recommendations expressed in this publication are those of the authors and do not necessarily reflect those of the U.S. Department of Justice.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflicts of interest.
Research involving human participants
Blood collection for this project was approved by the Institutional Review Board at the University at Albany. All blood donors used for this study supplied written consent, prior to blood donation, for the use of their blood for research purposes. This consent included the donors’ acknowledgement that they were healthy, over the age of 18, and not using any prescription or recreational drugs and that they could withdraw from the study at any time without any repercussions.
Funding
This project was supported by Awards No. 2011-DN-BX-K551, 2014-DN-BX-K016, and 2015-R2-CX-0021 awarded by the National Institute of Justice, Office of Justice Programs, U.S. Department of Justice (I.K.L.). The opinions, findings, and conclusions or recommendations expressed in this publication are those of the authors and do not necessarily reflect those of the U.S. Department of Justice.
Rights and permissions
About this article
Cite this article
Doty, K.C., McLaughlin, G. & Lednev, I.K. A Raman “spectroscopic clock” for bloodstain age determination: the first week after deposition. Anal Bioanal Chem 408, 3993–4001 (2016). https://doi.org/10.1007/s00216-016-9486-z
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00216-016-9486-z