Abstract
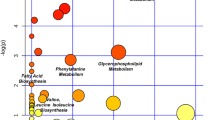
Osteoarthritis (OA), one of the most widespread musculoskeletal joint diseases among the aged, is characterized by the progressive loss of articular cartilage and continuous changes in subchondral bone. The exact pathogenesis of osteoarthritis is not completely clear. In this work, ultra-performance liquid chromatography coupled with quadrupole time-of-flight tandem mass spectrometry (UPLC/Q-TOF-MS) in combination with multivariate statistical analysis was applied to analyze the metabolic profiling of subchondral bone from 42 primary osteoarthritis patients. This paper described a modified two-step method for extracting the metabolites of subchondral bone from primary osteoarthritis patients. Finally, 68 metabolites were identified to be significantly changed in the sclerotic subchondral bone compared with the non-sclerotic subchondral bone. Taurine and hypotaurine metabolism and beta-alanine metabolism were probably relevant to the sclerosis of subchondral bone. Taurine, l-carnitine, and glycerophospholipids played a vital regulation role in the pathological process of sclerotic subchondral bone. In the sclerotic process, beta-alanine and l-carnitine might be related to the increase of energy consumption. In addition, our findings suggested that the intra-cellular environment of sclerotic subchondral bone might be more acidotic and hypoxic compared with the non-sclerotic subchondral bone. In conclusion, this study provided a new insight into the pathogenesis of subchondral bone sclerosis. Our results indicated that metabolomics could serve as a promising approach for elucidating the pathogenesis of subchondral bone sclerosis in primary osteoarthritis.

Metabolic analysis of osteoarthritis subchondral bone





Similar content being viewed by others
References
Hinman RS, Crossley KM. Patellofemoral joint osteoarthritis: an important subgroup of knee osteoarthritis. Rheumatology (Oxford). 2007;46(7):1057–62.
Pereira D, Ramos E, Branco J. Osteoarthritis. Acta Med Port. 2015;28(1):99–106.
Abramson SB, Attur M. Developments in the scientific understanding of osteoarthritis. Arthritis Res Ther. 2009;11(3):227.
Luo SX, Li S, Zhang XH, et al. Genetic polymorphisms of interleukin-16 and risk of knee osteoarthritis. PLoS One. 2015;10(5), e0123442.
Swift A. Osteoarthritis 1: physiology, risk factors and causes of pain. Nurs Times. 2012;108(7):12–5.
Loeser RF. Osteoarthritis year 2013 in review: biology. Osteoarthritis Cartilage. 2013;21(10):1436–42.
Scanzello CR, Loeser CR. Inflammatory activity in symptomatic knee osteoarthritis: not all inflammation is local. Arthritis Rheumatol. 2015. doi:10.1002/art.39304.
Sepriano A, Roman-Blas JA, Little RD, et al. DXA in the assessment of subchondral bone mineral density in knee osteoarthritis—a semi-standardized protocol after systematic review. Semin Arthritis Rheum. 2015. doi:10.1016/j.
Lories RJ, Luyten FP. The bone-cartilage unit in osteoarthritis. Nat Rev Rheumatol. 2011;7(1):43–9.
Zhen GH, Wen CY, Jia XF, Cao X. Inhibition of TGF-β signaling in mesenchymal stem cells of subchondral bone attenuates osteoarthritis. Nat Med. 2013;19(6):704–12.
Patti GJ, Yanes O, Siuzdak G. Innovation: metabolomics: the pogee of the omics trilogy. Nature reviews. Mol Cell Biol. 2012;13(4):263–9.
Zhang A, Sun H, Wang H. Serum metabolomics as a novel diagnostic approach for disease: a systematic review. Anal Bioanal Chem. 2012;404(4):1239–45.
Kang J, Zhu L, Lu J, Zhang X. Application of metabolomics in autoimmune diseases: insight into biomarkers and pathology. J Neuroimmunol. 2015;279:25–32.
Drexler DM, Reily MD, Shipkova PA. Advances in mass spectrometry applied to pharmaceutical metabolomics. Anal Bioanal Chem. 2011;399:2645–53.
Puchades CL, Pineda LA. Metabolomics in pharmaceutical research and development. Curr Opin Biotechnol. 2015;35:73–7.
Issaq HJ, Van QN, Waybright TJ, Muschik GM, Veenstra TD. Analytical and statistical approaches to metabolomics research. J Sep Sci. 2009;32(13):2183–99.
Schuhmacher R, Krska R, Weckwerth W, Goodacre R. Metabolomics and metabolite profiling. Anal Bioanal Chem. 2013;405:5003–50004.
Nordstrom A, O’Maille G, Qin C, Siuzdak G. Nonlinear data alignment for UPLC-MS and HPLC-MS based metabolomics: quantitative analysis of endogenous and exogenous metabolites in human serum. Anal Chem. 2006;78(10):3289–95.
Grauso L, Mariggio S, Corda D, Fontana A, Cutignano A. An improved UPLC-MS/MS platform for quantitative analysis of glycerophosphoinositol in mammalian cells. PLoS One. 2015;10(4), e0123198.
Adams SB, Setton LA, Nettles DL. The role of metabolomics in osteoarthritis research. J An Acad Orthop Surg. 2013;21(1):63–4.
Li X, Yang SY, Qiu YP, et al. Urinary metabolomics as a potentially novel diagnostic and stratification tool for knee osteoarthritis. Metabolomics. 2010;6:109–18.
Zhai GJ, Wang-Sattler R, Hart DJ, et al. Serum branched-chain amino acid to histidine ratio: a novel metabolomic biomarker of knee osteoarthritis. Ann Rheum Dis. 2010;69:1227–31.
Damyanovich AZ, Staples JR, Chan AD, Marshall KW. Comparative study of normal and osteoarthritic canine synovial fluid using 500 MHz 1H magnetic resonance spectroscopy. J Orthop Res. 1999;17(2):223–31.
Adams Jr SB, Setton LA, Kensicki E, Bolognesi MP, Toth AP, Nettles DL. Global metabolic profiling of human osteoarthritic synovium. Osteoarthr Cartil. 2012;20(1):64–7.
Mickiewicz B, Kelly JJ, Ludwig TE, et al. Metabolic analysis of knee synovial fluid as a potential diagnostic approach for osteoarthritis. J Orthop Res. 2015. doi:10.1002/jor.22949.
Altman R, Asch E, Bloch D, Bole G, Borenstein D. Development of criteria for the classification and reporting of osteoarthritis. Classification of osteoarthritis of the knee. Diagnostic and Therapeutic Criteria Committee of the American Rheumatism Association. Arthritis Rheum. 1986;29(8):1039–49.
Kellgren JH, Lawrence JS. Radiological assessment of osteoarthrosis. Ann Rheum Dis. 1957;16(4):494–502.
Want EJ, Masson P, Michopoulos F, Wilson ID. Global metabolic profiling of animal and human tissues via UPLC-MS. Nat Protoc. 2013;8(1):17–32.
Sangster T, Major H, Plumb R, Wilson AJ, Wilson ID. A pragmatic and readily implemented quality control strategy for HPLC–MS and GC–MS-based metabonomic analysis. Analyst (Cambridge, U K). 2006;131(10):1075–8.
Solberg R, Escobar J, Arduini A, et al. Metabolomic analysis of the effect of postnatal hypoxia on the retina in a newly born piglet model. PLoS One. 2013;8(6), e66540.
Bijlsma S, Bobeldijk I, Verheij ER, et al. Large-scale human metabolomics studies: a strategy for data (pre-) processing and validation. Anal Chem. 2006;78(2):567–74.
Max B, Mattias R. OPLS discriminant analysis: combining the strengths of PLS-DA and SIMCA classification. J Chemometrics. 2006;20:341–51.
Xia JG, David S. MetPA: a web-based metabolomics tool for pathway analysis and visualization. Bioinformatics. 2010;26(18):2342–4.
Gika HG, Theodoridis GA, Wilson ID. Liquid chromatography and ultra-performance liquid chromatography–mass spectrometry fingerprinting of human urine: sample stability under different handling and storage conditions for metabonomics studies. J Chromatogr A. 2008;1189(1–2):314–22.
Bylesjo M, Eriksson D, Trygg J. Orthogonal projections to latent structures as a strategy for microarray data normalization. BMC Bioinformatics. 2007;8:207.
Lajeunesse D, Reboul P. Subchondral bone in osteoarthritis: a biologic link with articular cartilage leading to abnormal remodeling. Curr Opin Rheumatol. 2003;15(5):628–33.
Sharif M, George E, Dieppe PA. Correlation between synovial fluid markers of cartilage and bone turnover and scintigraphic scan abnormalities in osteoarthritis of the knee. Arthritis Rheum. 1995;38(1):78–81.
Lamers RJ, DeGroot J, Spies-Faber EJ, et al. Identification of disease- and nutrient-related metabolic fingerprints in osteoarthritic Guinea pigs. J Nutr. 2003;133(6):1776–80.
Maher AD, Coles C, White J, et al. 1H NMR spectroscopy of serum reveals unique metabolic fingerprints associated with subtypes of surgically induced osteoarthritis in sheep. J Proteome Res. 2012;11(8):4261–8.
Mickiewicz B, Heard BJ, Chau JK. Metabolic profiling of synovial fluid in a unilateral ovine model of anterior cruciate ligament reconstruction of the knee suggests biomarkers for early osteoarthritis. J Orthop Res. 2014;33(1):71–7.
Okun JG, Kölker S, Schulze A, et al. A method for quantitative acylcarnitine profiling in human skin fibroblasts using unlabelled palmitic acid: diagnosis of fatty acid oxidation disorders and differentiation between biochemical phenotypes of MCAD deficiency. Biochim Biophys Acta. 2002;1584(2-3):91–8.
Seito N, Yamashita T, Tsukuda Y, et al. Interruption of glycerophospholipids synthesis enhances osteoarthritis development in mice. Arthritis Rheum. 2012;64(8):2579–88.
Fukumoto S, Iwamoto T, Sakai E, et al. Current topics in pharmacological research on bone metabolism: osteoclast differentiation regulated by glycosphingolipids. J Pharmacol Sci. 2006;100(3):195–200.
Ersek A, Xu K, Antonopoulos A, et al. Glycosphingolipid synthesis inhibition limits osteoclast activation and myeloma bone disease. J Clin Invest. 2015;125(6):2279–92.
Mansell JP, Collins C, Bailey AJ. Bone, not cartilage, should be the major focus in osteoarthritis. Nat Clin Pract Rheumatol. 2007;3(6):306–7.
Kosinska MK, Liebisch G, Lochnit G, et al. Sphingolipids in human synovial fluid—a lipidomic study. PLoS One. 2014;9(3), e91769.
Joql G, Tong L. Crystal structure of carnitine acetyltransferase and implications for the catalytic mechanism and fatty acid transport. Cell. 2003;112(1):113–22.
Nada MA, Rhead WJ, Sprecher H, Schulz H, Roe CR. Evidence for intermediate channeling in mitochondrial β-oxidation. J Biol Chem. 1995;270(2):530–5.
Frey JL, Li Z, Ellis JM, et al. Wnt-lrp5 signaling regulates fatty acid metabolism in the osteoblast. Mol Cell Biol. 2015;35(11):1979–91.
Colucci S, Mori G, Vaira S, et al. L-Carnitine and isovaleryl L-carnitine fumarate positively affect human osteoblast proliferation and differentiation in vitro. Calcif Tissue Int. 2005;76(6):458–65.
Ge P, Cui Y, Liu F, Luan J, Zhou X, Han J. L-carnitine affects osteoblast differentiation in NIH3T3 fibroblasts by the IGF-1/PI3K/Akt signalling pathway. Biosci Trends. 2015;9(1):42–8.
Orsal E, Halici Z, Bayir Y, et al. The role of carnitine on ovariectomy and inflammation-induced osteoporosis in rats. Exp Biol Med (Maywood). 2013;238(12):1406–12.
Abd-Allah AR, Al-Majed AA, Al-Yahya AA, Fouda SI, Al-Shabana OA. L-Carnitine halts apoptosis and myelosuppression induced by carboplatin in rat bone marrow cell cultures (BMC). Arch Toxicol. 2005;79(7):406–13.
Xie H, Tang SY, Liu H, et al. L-Carnitine protects against apoptosis of murine MC3T3-E1 osteoblastic cells. Amino Acids. 2008;35(2):419–23.
Patano N, Mancini L, Settanni MP, et al. L-Carnitine fumarate and isovaleryl-L-carnitine fumarate accelerate the recovery of bone volume/total volume ratio after experimentally induced osteoporosis in pregnant mice. Calcif Tissue Int. 2008;82(3):221–8.
Aydin A, Halici Z, Albayrak A, et al. Treatment with carnitine enhances bone fracture healing under osteoporotic and/or inflammatory conditions. Basic Clin Pharmacol Toxicol. 2015;117(3):173–9.
De Luca A, Pierno S, Camerino DC. Taurine: the appeal of a safe amino acid for skeletal muscle disorders. J Transl Med. 2015;13:243.
Lubec B, Ya-hua Z, Pertti S, Pentti T, Kitzmuller E, Lubec G. Distribution and disappearance of the radio labeled carbon derived from L-arginine and taurine in the mouse. Life Sci. 1997;60(26):2373–81.
Zhou C, Zhang X, Xu L, Wu T, Cui L, Xu D. Taurine promotes human mesenchymal stem cells to differentiate into osteoblast through the ERK pathway. Amino Acids. 2014;46(7):1673–80.
Park S, Kim H, Kim SJ. Stimulation of ERK2 by taurine with enhanced alkaline phosphatase activity and collagen synthesis in osteoblast-like UMR-106 cells. Biochem Pharmacol. 2001;62(6):1107–11.
Yuan LQ, Xie H, Luo XH, et al. Taurine transporter is expressed in osteoblasts. Amino Acids. 2006;31(2):157–63.
Chen SY, Yu HT, Kao JP, et al. An NMR metabolomic study on the effect of alendronate in ovariectomized mice. PLoS One. 2014;9(9), e106559.
Yuan LQ, Liu W, Cui RR, et al. Taurine inhibits osteoclastogenesis through the taurine transporter. Amino Acids. 2010;39(1):89–99.
Choi MJ, Chang KJ. Effect of dietary taurine and arginine supplementation on bone mineral density in growing female rats. Adv Exp Med Biol. 2013;776:335–45.
Hügle T, Kovacs H, Heijnen IA, et al. Synovial fluid metabolomics in different forms of arthritis assessed by nuclear magnetic resonance spectroscopy. Clin Exp Rheumatol. 2012;30(2):240–5.
Quesnele JJ, Laframboise MA, Wong JJ, Kim P, Wells GD. The effects of beta-alanine supplementation on performance: a systematic review of the literature. Int J Sport Nutr Exerc Metab. 2014;24(1):14–27.
Acknowledgments
This study was funded by National Natural Science Foundation of China (no. 89011060). The authors sincerely thank the subjects who participated in the study. The authors are also acknowledged for the sample collection at The First Affiliated Hospital of Chongqing Medical University, Chongqing, China.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
All the authors declare that they have no conflict of interest. The experiment designed for this study involved subchondral bone from 42 patients with primary osteoarthritis. The experiment was approved by the ethics committee of Chongqing Medical University. Written informed consent was obtained from each individual participant.
Additional information
Gang Yang and Hua Zhang contributed equally to this work.
Electronic supplementary material
Below is the link to the electronic supplementary material.
ESM 1
(PDF 456 kb)
Rights and permissions
About this article
Cite this article
Yang, G., Zhang, H., Chen, T. et al. Metabolic analysis of osteoarthritis subchondral bone based on UPLC/Q-TOF-MS. Anal Bioanal Chem 408, 4275–4286 (2016). https://doi.org/10.1007/s00216-016-9524-x
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00216-016-9524-x