Abstract
Objectives
To investigate the extent and pattern of off-label prescribing to children in primary care throughout Scotland.
Design
Assessment of prescribing to 167,865 children aged 0–16 years during the period November 1999 to October 2000 using data from 161 general practices using the national Scottish primary care computer system General Practice Administration System for Scotland.
Setting
One hundred and sixty one general practices in Scotland.
Results
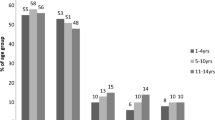
During the study period, at least one off-label prescription was issued to 17,715 (26.1%) children aged 0–16 years. Off-label prescribing due to lower than the recommended dose was the most common form of off-label prescribing (40–50%), with antibiotics and antihistamines making up the majority. Off-label prescribing due to higher than the recommended dose was also common (35% of all off-label prescribing), with antiasthmatics, topical corticosteroids and laxatives making up the majority. Off-label prescribing with respect to age was less common (6–16%) affecting mainly young children (less than 2 years old) and adolescents. Off-label prescribing with respect to formulation was the least common cause accounting for 5–10% of off-label prescribing.
Conclusions
This is the largest and most detailed study to date of paediatric off-label prescribing in primary care within the UK. Such off-label prescribing likely occurs as the result of several factors including a failure to update licensing information with currently accepted practice and confusion or unawareness of the licensing recommendations, further compounded by a lack of clinical trials data and suitable formulations for medicines commonly prescribed to young children and adolescents.
Similar content being viewed by others
References
Turner S, Gill A, Nunn T, Hewitt B, Choonara I (1996) Use of “off-label” and unlicensed drugs in paediatric intensive care unit. Lancet 347:549–550
Turner S, Longworth A, Nunn A, Choonara I (1998) Unlicensed and off-label drug use in paediatric wards: prospective study. BMJ 316:343–345
Conroy S, Choonara I, Impicciatore P, Mohn A, Arnell H, Rane A et al (2000) Survey of unlicensed and off-label drug use in paediatric wards in European countries. BMJ 320:79–82
Conroy S, McIntyre J, Choonara I (1999) Unlicensed and off-label drug use in neonates. Arch Dis Child Fetal Neonatal Ed 80:F142–F144
McIntyre J, Conroy S, Avery A, Corns H, Choonara I (2000) Unlicensed and off-label prescribing of drugs in general practice. Arch Dis Child 83:498–501
Chalumeau M, Treluyer JM, Salanave B, Assathiany R, Cheron G, Crocheton N et al (2000) Off-label and unlicensed drug use among French office based paediatricians. Arch Dis Child 83:502–505
Bucheler R, Schwab M, Morike K, Kalchthaler B, Mohr H, Schroder H et al (2002) Off-label prescribing to children in primary care in Germany: retrospective cohort study. BMJ 324:1311–1312
Schirm E, Tobi H, De Jong-Van Den Berg LT (2002) Unlicensed and off-label drug use by children in the community: cross sectional study. BMJ 324:1312–1313
’t Jong GW, Eland IA, Sturkenboom MC, van den Anker JN, Stricker BH (2002) Unlicensed and off-label prescription of drugs to children: population-based cohort study. BMJ 324:1313–1314
Committee for Proprietary Medicines (CPMP) (1997) Clinical investigation of medicinal products in children. 17-03-1997. Report CPMP/EWP/462/95. The European Agency for the Evaluation of Medicinal Products. Human Medicines Evaluation Unit, Canary Wharf, London
Impicciatore P, Choonara I (1999) Status of new medicines approved by the European Medicines Evaluation Agency regarding paediatric use. Br J Clin Pharmacol 48:15–18
’t Jong GW, Stricker BH, Choonar I, van den Anker JN (2002) Lack of effect of the European guidance on clinical investigation of medicines in children. Acta Paediatr 91:1233–1238
Ceci A, Felisi M, Catapano M, Baiardi P, Cipollina L, Ravera S et al (2002) Medicines for children licensed by the European Agency for the Evaluation of Medicinal Products. Eur J Clin Pharmacol 58:495–500
European Commission (2002) Better medicines for children. The European Commission. DG Enterprise. Unit F2-Paediatric Initiative, Brussels
Schirm E, Tobi H, De Jong-Van Den Berg LT (2003) Risk factors for unlicensed and off-label drug use in children outside the hospital. Pediatrics 111:291–295
Ufer M, Rane A, Karlsson A, Kimland E, Bergman U (2003) Widespread off-label prescribing of topical but not systemic drugs for 350,000 paediatric outpatients in Stockholm. Eur J Clin Pharmacol 58:779–783
Duncan R (1992) The electronic questionnaire—a software utility for both practice audit and national epidemiological studies. Primary Health Care Specialist Group Journal, J Inf Prim Health Care Feb:20–22
ABPI (1999) Compendium of data sheets and summaries of product characteristics. Datapharm Publications Limited, London
British Thoracic Society, National Asthma Campaign, Royal College of Physicians (London), et al. (1995) The British guidelines on asthma management: 1995 review and position statement. Thorax 52[Suppl 1]:S1–S20
CSM/MCA (2002) Inhaled corticosteroids and adrenal suppression in children. Curr Probl Pharmacovigilance 28:7
BPA/ABPI (1996) Licensing medicines for children. Royal Society of Paediatrics and Child Health, London
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Ekins-Daukes, S., Helms, P.J., Simpson, C.R. et al. Off-label prescribing to children in primary care: retrospective observational study. Eur J Clin Pharmacol 60, 349–353 (2004). https://doi.org/10.1007/s00228-004-0752-1
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00228-004-0752-1