Abstract
Objectives
To characterise the population of Alzheimer’s disease patients treated with acetylcholinesterase inhibitors, to analyse effectiveness and drug safety in the clinical practice, and to identify variables that may predict the response to therapy.
Methods
From September 2000 to December 2001, a total of 5,462 patients diagnosed with mild to moderate Alzheimer’s disease were enrolled at the time of their first prescription of the study drugs and followed up for an average of 10.5 months. Responders were defined as patients with a mini-mental state examination (MMSE) score improvement of 2 or more points from baseline after 9 months of therapy.
Results
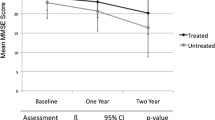
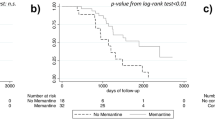
At 9 months, 2,853 patients (52.2%) completed the study. The mean change from baseline in MMSE scores was an improvement of 0.5 points (±3.0). The proportion of responders to the therapy was 15.7% at 9 months. A greater probability of response at 9 months was observed among patients without concomitant diseases at baseline [odds ratio (OR)=2.1, 95% confidence interval (CI) 1.5–2.9] and among those with a response at 3 months (OR=20.6, 95% CI 17.2–24.6). During the study period, 285 patients (5.2%) discontinued the treatment because of an adverse drug reaction.
Conclusions
Effectiveness of acetylcholinesterase inhibitors on cognitive symptoms of patients with mild to moderate Alzheimer’s disease is modest. At 9 months, improvement was evident only in a subgroup of patients without concomitant diseases and who had demonstrated a response at 3 months.

Similar content being viewed by others
References
Scarpini E, Scheltens P, Feldman H (2003) Treatment of Alzheimer’s disease: current status and new perspectives. Lancet Neurol 2:539–547
Lanctôt KL, Herrmann N, Yau KK et al (2003) Efficacy and safety of cholinesterase inhibitors in Alzheimer’s disease: a meta-analysis. CMAJ 169:557–564
Rogers SL, Fralow MR, Doody RS, Mohs R, Friedhoff LT, Donepezil Study Group (1998) A 24-week, double-blind, placebo-controlled trial of donepezil in patients with Alzheimer’s disease. Neurology 50:136–145
Rosler M, Anand R, Cicin-Sain A et al (1999) Efficacy and safety of rivastigmine in patients with Alzheimer’s disease: international randomised controlled trail. BMJ 318:633–640
Wilcock GK, Lilienfeld S, Gaens E on behalf on the Galantamine International-1 Study Group (2000) Efficacy and safety of galantamine in patients with mild to moderate Alzheimer’s disease: multicentre randomised controlled trial. BMJ 321:1–7
Peripheral and Central Nervous System Drugs Advisory Committee Meeting, July 7, 1989. Department of Health and Human Services, Public Health Service, Food and Drug Administration, Rockville, MD, p 227
Aricept® (donepezil hydrochloride) (1997) Summary of product characteristics. Pfizer, Italy
Exelon® (rivastigmine) (1998) Summary of product characteristics. Novartis, Italy
Reminyl® (galantamine) (2001) Summary of product characteristics. Janssen, Italy
Government of Italy. Protocollo di monitoraggio dei piani di trattamento farmacologico per la malattia di Alzheimer. D M July 20, 2000. Gazzetta Ufficiale della Repubblica Italiana n. 204, 1 Settembre 2000
Ray WA (2003) Evaluating medication effects outside of clinical trials: new-user designs. Am J Epidemiol 158:915–920
McKhann G, Drachman D, Folstein M, Katzman R, Price D, Stadlan EM (1984) Clinical diagnosis of Alzheimer’s disease: report of the NINCDS-ADRDA Work Group under the auspices of the Department of Health and Human Services Task Force on Alzhemeir’s disease. Neurology 34:939–944
Folstein M, Folstein S, Mc Hugh PR (1975) Mini-Mental State: a practical method for grading the cognitive state of patients for the clinician. J Psychiatr Res 12:189–198
Katz S, Ford AB, Moskowitz RW, Jackson BA, Jaffe MW (1963) Studies of illness in the aged. The index of ADL: a standardized measure of biological and psychological function. JAMA 185:914–919
Lawton MP, Brody EM (1969) Assessment of older people: self-maintaining and instrumental activities of daily living. Gerontologist 9:179–186
Doraiswamy PM, Bieber F, Kaiser L, Krishnan KR, Reuning-Scherer J, Gulanski B (1997) Patterns and predictors of baseline cognitive performance in multicenter Alzheimer’s disease trials. Neurology 48:1511–1517
Winblad B, Brodaty H, Gauthier S et al (2001) Pharmacotherapy of Alzheimer’s disease: is there a need to redefine treatment success? Int J Geriatr Psychiatry 16:653–666
AD2000 Collaborative Group (2004) Long-term donepezil treatment in 565 patients with Alzheimer’s disease (AD2000): randomized double blind trial. Lancet 363:2105–2115
Winblad B, Engedal K, Soininen H et al (2001) A 1-year, randomized, placebo-controlled study of donepezil in patients with mild to moderate AD. Neurology 57:489–495
Imbimbo BP (2001). Pharmacodynamic-Tolerability relationships of cholinesterase inhibitors for Alzheimer’s disease. CNS Drugs 15:375–390
National Institute for Clinical Excellence. Guidance on the Use of Donepezil, Rivastigmine and Galantamine for the Treatment of Alzheimer’s disease. Technology Appraisal Guidance—No. 19. Available at: http://www.nice.org.uk (Accessed October 4, 2004)
Acknowledgements
We thank Umberto Senin (Perugia University), Ovidio Brignoli (General Practitioner), and Teresa Di Fiandra (Ministry of Health) for supervising the study; Francesca Ravaioli (Ministry of Health) for coordinating data collection; Monica Bolli and Paola Ruggeri (Istituto Superiore di Sanità) for data collection; Clara Bianchi, Roberto Da Cas, Francesca Menniti Ippolito, Stefania Spila Alegiani, and Giuseppe Traversa (Istituto Superiore di Sanità) who discussed the study findings. Expenses were covered by National Health Service funds.
Author information
Authors and Affiliations
Corresponding author
Additional information
The list of Alzheimer’s disease unit investigators is reported in the Appendix
Appendices
Appendix
Alzheimer’s disease unit investigators
Abruzzo: Gabriele A. Servizio Neurologia, Sulmona (AQ)—Lechiara MC. Presidio Ospedaliero “S. Rinaldi”, Pescina (AQ). Basilicata: Paciello M. UVA Ospedale San Carlo, Potenza. Calabria: Ambrosio L. ASL4 Ospedale Annunziata, Cosenza; Buccomino D. Centro Salute Mentale, Roggiano Gravina (CS)—Carabetta V. ASL9 Locri (RC)—Filastro F. Centro Alzheimer ASL7, Girifalco (CZ)—Lamenza F. Centro Demenze ASL3, Rossano (CS)—Bruzzese T. Centro Salute Mentale ASL9, Locri (RC). Campania: Caterino L. Coordinamento UVA ASL CE/2, Aversa (CE)—Cerqua G. Distretto 40, Castelvolturno (CE)—D’amore A. Distretto 37, Casal di Principe (CE) e Distretto 35—Carinaro (CE)—De Martino G. ASL NA/5, Castellammare di Stabia (NA)—Di Fusco A. Distretto 38, S. Maria Capua Vetere (CE)—Di Sato G. Dipartimento Medicina Interna e Specialistica ASL BN/1, Cerreto Sannita (BN)—Feleppa MAO. Rummo, Benevento—Ianniello P. Distretto 39, Capua (CE)—Maiello M. Distretto 43, Sessa Aurunca (CE)—Iazeolla M. Distretto 17, Benevento, Distretto 18, Cautano (BN), Distretto 22, Morcone (BN) e UOAA Distretto 19, Montesarchio (BN)—Maio G. UOAA Distretto 23, S. Bartolomeo in Galdo (BN)—Marino MP. Distretto 20, S. Agata dei Goti (BN) e Distretto 24, S. Giorgio del Sannio (BN)—Nuzzo P. Distretto 42, Mondragone (CE)—Femina G. Centro Alzheimer ASL AV2, Atripalda (AV)—Roma M. Distretto 36, Frignano (CE)—Santagata P. Distretto 21, Telese Terme (BN)—Schipani G. UOAA Distretto 102, Battipaglia (SA) e Distretto 97, Salerno—Scognamiglio P. Distretto 41, Pignataro Maggiore (CE).
Emilia Romagna: Alberti M. Centro Demenze, Guastalla (RE)—Arena C. Poliambulatorio, San Lazzario di Savena (BO)—Boiardi R. Centro Distrettuale Demenze, Castelnovo Ne’ Monti (RE)—Ferrari P. Ospedale, Scandiano (RE)—Ghidoni E. UVA Arcispedale Santa Maria Nuova, Reggio Emilia e AUSL Centro Distrettuale Disturbi Cognitivi, Reggio Emilia—Lucchetti L. Consultorio Aziendale per i Disturbi Cognitivi, AUSL Piacenza—Miconi G. Ambulatorio Consulenza Geriatria AUSL Bologna Sud, Porretta Terme (BO)—Pellati M. Centro Distrettuale Demenze, AUSL Reggio Emilia, Correggio (RE)—Roberti R. Centro Demenze, Montecchio (RE)—Friuli Venezia Giulia: Prati P. ASL4 Ospedale Gervasutta, Udine—Lazio: Alfonsi S. ASL Frosinone Distretto C Dipartimento Salute Mentale, Sora (FR)—Giramma F. IPFD Venditti, Centro Provinciale UVA, Ospedale S. Maria Goretti, Latina—Di Cioccio L. UOC di Geriatria e Centro UVA Servizio Geriatrico, Aquino (FR)—Lancetti USL Ospedale Bel Colle, Viterbo. Liguria: Carniglia De Carli R. ASL4 Pres.San. Territoriale, Chiavari (GE)—Colameo A. Unità Geriatrica di Cura per le Demenze ASL5 Spezzino, Sarzana—Montanari GP. Neurologia Geriatria USL5 Spezzino, La Spezia. Lombardia: Alberoni M. UVA IRCCS S. Maria Nascente, Fondazione Don Gnocchi, Milano—Bargnani C. Clinica S. Rocco di Franciacorta, Ome (BS)—Boffelli S. UO Geriatria, Ospedale Poliambulanza, Brescia—Bosio, Clinica S. Anna, Neurologia, Brescia—Bugiani, Ospedale Besta, Milano—Casale R. ISP Maugeri, Clinica del Lavoro, Montescano (PV)—Chia F. UVA AO Mellino Mellini, Chiari (BS)—Clerici F. UVA Clinica Neurologica Ospedale L. Sacco, Milano—Cuzzoni G. IPAB Istituto S. Margherita, S. Margherita (PV)—Farina PM. IPAB Pii Istituti Unificati, Belgioioso (PV)—Franzoni S. Ospedale “Richiedei”, Gussago (BS)—Gerini Niguarda CA’ Granda, Milano—Magnani G. Divisione di Neurologia Ospedale San Raffaele, Milano—Magrotti E. Neurologia ASL Pavia, Pavia—Padovani A. Spedali Civili, Brescia—Ranzenigo A. UO Geriatria Ospedale S. Orsola Fatebenefratelli, Brescia—Sacilotto G. Istituti Clinici Perfezionamento, Milano – Sinforiani, Istituto Neurologico Mondino, Pavia—Spinnler H. Clinica Neurologica III Ospedale S. Paolo, Milano—Viti N. CDC Istituto Palazzolo, Fondazione Don Carlo Gnocchi, Milano—Zanetti O. UO Alzheimer IRCCS Centro S. Orsola Fatebenefratelli, Brescia. Marche: Livini L. Distretto Centro UVA, ASL 11, Fermo—Masotti, AUSL6, Fabriano (AN)—Molise: Cuccaro, ASL3 “Centro Molise”, Campobasso. Piemonte: Diarassouba A. Az. Regionale ASL7, Chivasso (TO)—Francesconi M. ASL19, Asti—Guala A. ASL12, Osp. Infermi, Divisione Geriatria, Biella—Infantino C. Az. Regionale USL5, Collegno, Rivoli (TO)—Seliak D. ASL17 UOA Neurologia, Savigliano (CN). Provincia autonoma di Bolzano: Gasperi A. Servizio Neurologia Azienda Sanitaria, Brunico (BZ). Puglia: De Matteis C. ASL LE/1 Geriatria P O S.Giuseppe da Copertino, Copertino (LE)—Elia A. ASL LE/1, Lecce—Fulgido ASL LE/1 UVA Galatina, Galatina (LE)—Totaro G. Ospedale S. Michele ASL Cerignola, Monte S. Angelo (FG). Sardegna: Capelli P. UVA, Geriatria Ospedale San Francesco ASL3, Nuoro—Cosseddu G. ASL2 Ospedale S. Giovanni di Dio, Olbia (SS)—Minnai G. ASL6 Ospedale S. Martino, Oristano. Sicilia: Arena MG. Clinica Neurologica, Policlinico Universitario, Messina—Di Pasquale MR. Neurologia Ospedale Piemonte, Messina—Emilici A. Dipartimento Salute Mentale, Patti (ME)—Lalicata L. ASL AG/1 Centro Salute Mentale, Canicattì (AG)—Nastasi G. Ospedale Papardo Neurologia, Messina—Scifo E. USL1, Agrigento—Xerra AM. ASL ME/5 Centro di Salute Mentale, Milazzo (ME). Toscana: Cipriani G. Neurologia Ospedale “Versilia”, Viareggio (LU). Umbria: Mecocci P. Geriatria Policlinico Monteluce, Perugia—Parnetti L. Neurologia Policlinico Monteluce, Perugia—Pollioni F. ASL2 S.M. Angeli, Assisi—Ricci S. ASL2 Ambulatorio Neurologico di Bastia Umbra (PG), Ellera (PG), Castiglione del Lago (PG), Città della Pieve (PG), Ponte San Giovanni (PG) e Serv. Mal. Cerebrovasc. e Neurol, Perugia. Valle d’Aosta: Bottacchi E. UB Neurologia e Neurofisiopatologia, Ospedale Regionale della Valle d’Aosta, Aosta. Veneto: Cester A. Geriatria Ospedale, Dolo (VE) e Geriatria Ospedale, Noale Mirano (VE)—Garonna F. ASL3 Psichiatria Ospedale, Bassano del Grappa (VI)—Zanetti L. UO Geriatria Ospedale di Conegliano, Pieve di Soligo (TV).
Rights and permissions
About this article
Cite this article
Raschetti, R., Maggini, M., Sorrentino, G.C. et al. A cohort study of effectiveness of acetylcholinesterase inhibitors in Alzheimer’s disease. Eur J Clin Pharmacol 61, 361–368 (2005). https://doi.org/10.1007/s00228-005-0946-1
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00228-005-0946-1