Abstract
Objective
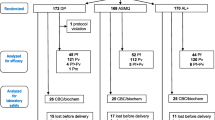
To determine the pharmacokinetic properties of artemether and lumefantrine (AL) in pregnant women with recrudescent uncomplicated multi-drug resistant falciparum malaria.
Methods
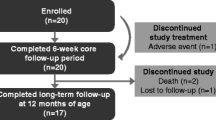
Pregnant women who had recurrence of parasitaemia following 7 days supervised quinine treatment were treated with AL. Serial blood samples were taken over a 7-day period, and pharmacokinetic parameters were estimated. For lumefantrine, these data were compared in a population pharmacokinetic model with data from non-pregnant, mainly male adults with acute malaria.
Results
The pregnant women (five in the second trimester and eight in the third trimester) had lower concentrations of artemether, dihydroartemisinin and lumefantrine, and the elimination of lumefantrine in pregnant women was more rapid than reported previously in non-pregnant adults.
Conclusion
Pregnancy is associated with reduced plasma concentrations of both artemether and lumefantrine. This is likely to be of therapeutic significance as plasma concentrations of lumefantrine, after elimination of artemether, are an important determinant of cure. Further studies are needed to determine the optimum dose regimen of artemether-lumefantrine in pregnancy.




Similar content being viewed by others
References
McGready R, Stepniewska K, Edstein MD, Cho T, Gilveray G, Looareesuwan S, White NJ, Nosten F (2003a) The pharmacokinetics of atovaquone and proguanil in pregnant women with acute falciparum malaria. Eur J Clin Pharmacol 59: 545
McGready R, Stepniewska K, Seaton E, Cho T, Cho D, Ginsberg A, Edstein MD, Ashley E, Looareesuwan S, White NJ, Nosten F (2003b) Pregnancy and use of oral contraceptives reduces the biotransformation of proguanil to cycloguanil. Eur J Clin Pharmacol 59:553
McGready R, Stepniewska K, Ward SA, Cho T, Gilvary G, Looareesuwan S, White NJ, Nosten F (2006) Pharmacokinetics of dihydroartemisinin following oral artesunate treatment of pregnant women with acute uncomplicated falciparum malaria. Eur J Clin Pharmacol 62:367–371
Nosten F, van Vugt M, Price R, Luxemburger C, Thway KL, Brockman A, McGready R, ter Kuile F, Looareesuwan S, White NJ (2000) Effects of artesunate-mefloquine combination on incidence of Plasmodium falciparum malaria and mefloquine resistance in western Thailand: a prospective study. Lancet 356:297
Nosten F, Vincenti M, Simpson J, Yei P, Thwai KL, de Vries A, Chongsuphajaisiddhi T, White NJ (1999) The effects of mefloquine treatment in pregnancy. Clin Infect Dis 28:808
McGready R, Cho T, Samuel, Villegas L, Brockman A, van Vugt M, Looareesuwan S, White NJ, Nosten F (2001) Randomized comparison of quinine-clindamycin versus artesunate in the treatment of falciparum malaria in pregnancy. Trans R Soc Trop Med Hyg 95:651
WHO (2003) Assessment of the safety of artemisinin compounds in pregnancy. World Health Organization, Geneva
McGready R, Cho T, Keo NK, Thwai KL, Villegas L, Looareesuwan S, White NJ, Nosten F (2001) Artemisinin antimalarials in pregnancy: a prospective treatment study of 539 episodes of multidrug-resistant Plasmodium falciparum. Clin Infect Dis 33:2009
Vugt M van, Looareesuwan S, Wilairatana P, McGready R, Villegas L, Gathmann I, Mull R, Brockman A, White NJ, Nosten F (2000) Artemether-lumefantrine for the treatment of multidrug-resistant falciparum malaria. Trans R Soc Trop Med Hyg 94:545
Longo M, Zanoncelli S, Manera D, Brughera M, Colombo P, Lansen J, Mazue G, Gomes M, Taylor WR, Olliaro P (2006) Effects of the antimalarial drug dihydroartemisinin (DHA) on rat embryos in vitro. Reprod Toxicol 21:83
Wang TY (1989) Follow-up observation on the therapeutic effects and remote reactions of artemisinin (Qinghaosu) and artemether in treating malaria in pregnant woman. J Tradit Chin Med 9:28
Adam I, Elwasila E, Mohammed Ali DA, Elansari E, Elbashir MI (2004) Artemether in the treatment of falciparum malaria during pregnancy in eastern Sudan. Trans R Soc Trop Med Hyg 98:509
Sowunmi A, Oduola AMJ, Ogundahunsi O, Fehintola FA, Ilesanmi OA, Akinyinka OO, Arowojolu AO (1998) Randomised trial of artemether versus artemether and mefloquine for the treatment of chloroquine/sufadoxine-pyrimethamine-resistant falciparum malaria during pregnancy. J Obstet Gynaecol 18: 322
White NJ, van Vugt M, Ezzet F (1999) Clinical pharmacokinetics and pharmacodynamics and pharmacodynamics of artemether-lumefantrine. Clin Pharmacokinet 37:105
Dubowitz LM, Dubowitz V, Goldberg C (1970) Clinical assessment of gestational age in the newborn infant. J Pediatr 77:1
McGready R, Simpson J, Panyavudhikrai S, Loo S, Mercuri E, Haataja L, Kolatat T, Nosten F, Dubowitz L (2000) Neonatal neurological testing in resource-poor settings. Ann Trop Paediatr 20:323
Haataja L, McGready R, Arunjerdja R, Simpson JA, Mercuri E, Nosten F, Dubowitz L (2002) A new approach for neurological evaluation of infants in resource-poor settings. Ann Trop Paediatr 22:355
Brockman A, Paul RE, Anderson TJ, Hackford I, Phaiphun L, Looareesuwan S, Nosten F, Day KP (1999) Application of genetic markers to the identification of recrudescent Plasmodium falciparum infections on the northwestern border of Thailand. Am J Trop Med Hyg 60:14
Lindegardh N, Annerberg A, Blessborn D, Bergqvist Y, Day N, White NJ (2005) Development and validation of a bioanalytical method using automated solid-phase extraction and LC-UV for the simultaneous determination of lumefantrine and its desbutyl metabolite in plasma. J Pharm Biomed Anal 37:081
Souppart C, Gauducheau N, Sandrenan N, Richard F (2002) Development and validation of a high-performance liquid chromatography-mass spectrometry assay for the determination of artemether and its metabolite dihydroartemisinin in human plasma. J Chromatogr B Analyt Technol Biomed Life Sci 774:195
Ezzet F, van Vugt M, Nosten F, Looareesuwan S, White NJ (2000) Pharmacokinetics and pharmacodynamics of lumefantrine (benflumetol) in acute falciparum malaria. Antimicrob Agents Chemother 44:697
Ashley E, Stepniewska K, Lindergardh N, McGready R, Annerberg A, Hutagalung R, Singtoroj T, Hla G, Brockman A, Proux S, Wilahphaingern J, Singhasivanon P, White N, Nosten F (2006) A pharmacokinetic study of artemether-lumefantrine given once daily for the treatment of uncomplicated multidrug resistant falciparum malaria. Trop Med Int Health (in press)
Lefevre G, Looareesuwan S, Treeprasertsuk S, Krudsood S, Silachamroon U, Gathmann I, Mull R, Bakshi R (2001) A clinical and pharmacokinetic trial of six doses of artemether-lumefantrine for multidrug-resistant Plasmodium falciparum malaria in Thailand. Am J Trop Med Hyg 64:247
Brockman A, Price RN, van Vugt M, Heppner DG, Walsh D, Sookto P, Wimonwattrawatee T, Looareesuwan S, White NJ, Nosten F (2000) Plasmodium falciparum antimalarial drug susceptibility on the north-western border of Thailand during five years of extensive use of artesunate-mefloquine. Trans R Soc Trop Med Hyg 94:537
Teja-Isavadharm P, Nosten F, Kyle DE, Luxemburger C, ter Kuile F, Peggins JO, Brewer TG, White NJ (1996) Comparative bio-availability of oral, rectal and intramuscular artemether in healthy subjects-use of simultaneous measurement by high performance liquid chromatography with electro-chemical detection and bioassay. Brit J Clin Pharmacol 42:599–604
Teja-Isavadharm P, Peggins JO, Brewer TG, White NJ, Webster HK, Kyle DE (2004) Plasmodium falciparum-based bioassay for measurement of artemisinin derivatives in plasma or serum. Antimicrob Agents Chemother 48:954
Ezzet F, Mull R, Karbwang J (1998) Population pharmacokinetics and therapeutic response of CGP 56697 (artemether + benflumetol) in malaria patients. Br J Clin Pharmacol 46:553
Herrera E, Amusquivar E, Lopez-Soldado I, Ortega H (2006) Maternal lipid metabolism and placental lipid transfer. Horm Res 65:59
Price R, Uhlemann AC, van Vugt M, Brockman A, Hutagalung R, Nair S, Nash D, Singhasivanon P, Anderson T, Krishna S, White NJ, Nosten F (2006) Molecular and pharmacological determinants of the therapeutic response to artemether-lumefantrine in multi-drug resistant falciparum malaria. Clin Infect Dis 42:1570–1577
Acknowledgements
We sincerely thank the pregnant women for their cooperation in completing this study. We thank also the diligent staff from SMRU. The Shoklo Malaria Research Unit is part of the Faculty of Tropical Medicine in Bangkok. This investigation was part of the Wellcome-Trust Mahidol University Oxford Tropical Medicine Research Programme, supported by the Wellcome-Trust of Great Britain. The study was supported by UNDP/World Bank/WHO Special Programme for Research and Training in Tropical Diseases (TDR).
Author information
Authors and Affiliations
Corresponding author
Additional information
An erratum to this article can be found online at http://dx.doi.org/http://dx.doi.org/10.1007/s00228-009-0671-2.
Rights and permissions
About this article
Cite this article
McGready, R., Stepniewska, K., Lindegardh, N. et al. The pharmacokinetics of artemether and lumefantrine in pregnant women with uncomplicated falciparum malaria. Eur J Clin Pharmacol 62, 1021–1031 (2006). https://doi.org/10.1007/s00228-006-0199-7
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00228-006-0199-7