Abstract
Background
The diffusion of innovations model proposes that early adopters of innovation influence others. This study was undertaken to determine if early prescribers and users of newly marketed drugs had different sociodemographic and professional characteristics as compared to majority and late users and prescribers.
Methods
After market availability in Manitoba, Canada, of celecoxib, alendronate, clopiodogrel and pantoprazole, time to first prescriptions was determined. Early, majority and late adopters of the new drug were characterized by this diffusion time. The prescription, health and prescriber records were compared across adopter categories. The likelihood of being an early or late prescriber or user of the new medications according to patient demographic characteristics, physician factors (specialty and place of training) and neighborhood income was determined with polytomous logistic regression.
Results
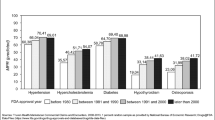
Celecoxib demonstrated a much more rapid uptake into routine use than the other drugs. More than 300 Manitoba physicians prescribed celecoxib within two weeks of market availability. Early prescribers of celecoxib were more likely than majority prescribers to be general practitioners (OR = 1.81, 95%CI: 1.40–2.35) and have hospital affiliations (OR = 1.35, 95%CI: 1.03–1.77). Early users of celecoxib were more likely than the majority to have arthritic conditions, have a high income and have paid out-of-pocket for their prescription. For alendronate, clopidogrel and pantoprazole, only prescription drug coverage predicted adopter category. Early prescribers of one new drug were not early prescribers of the other new drugs.
Conclusion
No common group of patients or physicians who were early prescribers or users of all four medications was described.

Similar content being viewed by others
References
Britten N, Ukoumunne O (6-12-1997) The influence of patients’ hopes of receiving a prescription on doctors’ perceptions and the decision to prescribe: a questionnaire survey. BMJ 315:1506–1510
Therapeutics Initiative (1999) Celecoxib (Celebrex) Is it a breakthrough drug? Therapeutics Letter August/September:1–2
Bull SA, Conell C, Campen DH (2002) Relationship of clinical factors to the use of Cox-2 selective NSAIDs within an arthritis population in a large HMO. J Manag Care Pharm 8:252–258
Cox ER, Motheral B, Frisse M, Behm A, Mager D (2003) Prescribing COX-2s for patients new to cyclo-oxygenase inhibition therapy. Am J Manag Care 9:735–742
Fitt NS, Mitchell SL, Cranney A, Gulenchyn K, Huang M, Tugwell P (20-3-2001) Influence of bone densitometry results on the treatment of osteoporosis. CMAJ 164:777–781
Goldstein LB, Bonito AJ, Matchar DB, Duncan PW, DeFriese GH, Oddone EZ, Paul JE, Akin DR, Samsa GP (1995) US national survey of physician practices for the secondary and tertiary prevention of ischemic stroke. Design, service availability, and common practices. Stroke 26:1607–1615
Hungin AP, Rubin GP, O’Flanagan H (1999) Long-term prescribing of proton pump inhibitors in general practice. Br J Gen Pract 49:451–453
Jaglal SB, McIsaac WJ, Hawker G, Jaakkimainen L, Cadarette SM, Chan BT (31-10-2000) Patterns of use of the bone mineral density test in Ontario, 1992–1998. CMAJ 163:1139–1143
Quartero AO, Smeets HM, de Wit NJ (2003) Trends and determinants of pharmacotherapy for dyspepsia: analysis of 3-year prescription data in The Netherlands. Scand J Gastroenterol 38:676–677
Solomon CG, Connelly MT, Collins K, Okamura K, Seely EW (2000) Provider characteristics: impact on bone density utilization at a health maintenance organization. Menopause 7:391–394
Solomon DH, Levin E, Helfgott SM (2000) Patterns of medication use before and after bone densitometry: factors associated with appropriate treatment. J Rheumatol 27:1496–1500
Solomon DH, Schneeweiss S, Glynn RJ, Levin R, Avorn J (15-12-2003) Determinants of selective cyclooxygenase-2 inhibitor prescribing: are patient or physician characteristics more important? Am J Med 115:715–720
Rogers EM (1995) Diffusion of innovations
Greer AL (1988) The state of the art versus the state of the science. The diffusion of new medical technologies into practice. Int J Technol Assess Health Care 4:5–26
Hayward RS, Guyatt GH, Moore KA, McKibbon KA, Carter AO (1997) Canadian physicians’ attitudes about and preferences regarding clinical practice guidelines. Can Med Assoc J 156:1715–1723
Kanouse DE, Jacoby I (1988) When does information change practitioners’ behavior? Int J Technol Assess Health Care 4:27–33
McKinlay JB (1981) From “promising report” to “standard procedure”: seven stages in the career of a medical innovation. Milbank Memorial Fund Quarterly/Health and Society 59:374–411
Miké V, Krauss AN, Ross GS (1998) Responsibility for clinical innovation: a case study in neonatal medicine. Eval Health Prof 21:3–26
Robert G, Gabbay J, Stevens A (1998) Which are the best information sources for identifying emerging health care technologies? An international Delphi survey. Int J Technol Assess Health Care 14:636–643
Steffensen FH, Sorensen HT, Olesen F (1999) Diffusion of new drugs in Danish general practice. Fam Pract 16:407–413
Tamblyn R, McLeod P, Hanley JA, Girard N, Hurley J (2003) Physician and practice characteristics associated with the early utilization of new prescription drugs. Med Care 41:895–908
Dybdahl T, Andersen M, Sondergaard J, Kragstrup J, Kristiansen IS (2004) Does the early adopter of drugs exist? A population-based study of general practitioners’ prescribing of new drugs. Eur J Clin Pharmacol 60: 667–672
Anderson DM, Needle RH, Mosow SR (1986) Diffusion of innovations in health promotion: a microcomputer-enhanced program for children. Fam Commu Health 9:27–36
Kozyrskyj AL, Mustard CA (1998) Validation of an electronic, population-based prescription database. Ann Pharmacother 32:1152–1157
Robinson JR, Young TK, Roos LL, Gelskey DE (1997) Estimating the burden of disease. Comparing administrative data and self-reports. Med Care 35:932–947
Lix LM, Yogendran M, Burchill C et al (2006) Defining and validating chronic diseases: an administrative data approach. Manitoba Centre for Health Policy, Winnipeg
Menec VH, Black C, Roos NP, Bogdanovic B, Reid R (1999) Defining practice populations for primary care: methods and issues. Manitoba Centre for Health Policy, Winnipeg
Buban GM, Link BK, Doucette WR (15-2-2001) Influences on oncologists’ adoption of new agents in adjuvant chemotherapy of breast cancer. J Clin Oncol 19:954–959
Groves KEM, Flanagan PS, MacKinnon NJ (2002) Why physicians start or stop prescribing a drug: literature review and formulary implications. Formulary 37:186–194
Jones MI, Greenfield SM, Bradley CP (18-8-2001) Prescribing new drugs: qualitative study of influences on consultants and general practitioners. BMJ 323:378–381
Peay MY, Peay ER (1994) Innovation in high risk drug therapy. Soc Sci Med 39:39–52
Prosser H, Walley T (2003) New drug uptake: qualitative comparison of high and low prescribing GPs’ attitudes and approach. Fam Pract 20:583–591
Wieringa NF, Denig P, de Graeff PA, Vos R (2001) Assessment of new cardiovascular drugs. Relationships between considerations, professional characteristics, and prescribing. Int J Technol Assess Health Care 17:559–570
Tamblyn R, Laprise R, Hanley JA, Abrahamowicz M, Scott S, Mayo N, Hurley J, Grad R, Latimer E, Perreault R, McLeod P, Huang A, Larochelle P, Mallet L (24-1-2001) Adverse events associated with prescription drug cost-sharing among poor and elderly persons. JAMA 285:421–429
Coleman J, Menzel H, Katz E (1966) Medical innovation–a diffusion study. Bobbs-Merrill, Indianapolis
Weiss R, Charney E, Baumgardner RA, German PS, Mellits ED, Skinner EA, Williamson JW (1990) Changing patient management: what influences the practicing pediatrician? Pediatrics 85:791–795
Prosser H, Almond S, Walley T (2003) Influences on GPs’ decision to prescribe new drugs-the importance of who says what. Fam Pract 20:61–68
Pugh MJ, Anderson J, Pogach LM, Berlowitz DR (2003) Differential adoption of pharmacotherapy recommendations for type 2 diabetes by generalists and specialists. Med Care Res Rev 60:178–200
Majumdar SR, McAlister FA, Soumerai SB (15-10-2003) Synergy between publication and promotion: comparing adoption of new evidence in Canada and the United States. Am J Med 115:467–472
Fendrick AM, Hirth RA, Chernew ME (1996) Differences between generalist and specialist physicians regarding Helicobacter pylori and peptic ulcer disease. Am J Gastroenterol 91:1544–1548
CBC News (2002) Targeting doctors. http://www.cbc.ca/disclosure/archives/0103_pharm/docs/totalpromodollars.pdf
Mintzes B, Barer ML, Kravitz RL, Kazanjian A, Bassett K, Lexchin J, Evans RG, Pan R, Marion SA (2002) Influence of direct to consumer pharmaceutical advertising and patients’ requests on prescribing decisions: two site cross sectional survey. Br Med J 324:278–279
Behan K, Cutts C, Tett SE (2005) Uptake of new drugs in rural and urban areas of Queensland, Australia: the example of COX-2 inhibitors. Eur J Clin Pharmacol 61:55–58
Soumerai SB, McLaughlin TJ, Gurwitz JH, Guadagnoli E, Hauptman PJ, Borbas C, Morris N, McLaughlin B, Gao X, Willison DJ, Asinger R, Gobel F (6-5-1998) Effect of local medical opinion leaders on quality of care for acute myocardial infarction: a randomized controlled trial. JAMA 279:1358–1363
Jones MI, Greenfield SM, Jowett S, Bradley CP, Seal R (2001) Proton pump inhibitors: a study of GPs’ prescribing. Fam Pract 18:333–338
McGettigan P, Golden J, Fryer J, Chan R, Feely J (2001) Prescribers prefer people: the sources of information used by doctors for prescribing suggest that the medium is more important than the message. Br J Clin Pharmacol 51:184–189
Ruof J, Mittendorf T, Pirk O, der Schulenburg JM (2002) Diffusion of innovations: treatment of Alzheimer’s disease in Germany. Health Policy 60:59–66
Helin-Salmivaara A, Huupponen R, Virtanen A, Klaukka T (2005) Adoption of celecoxib and rofecoxib: a nationwide database study. J Clin Pharm Ther 30:145–152
Acknowledgement
Data for this research were accessed from the Population Health Research Data Repository at the Manitoba Centre for Health Policy. The research was funded through an unrestricted grant from Pfizer (formerly Pharmacia) Inc. Pfizer Inc did not contribute to the research design, data analysis or manuscript writing, and was unaware of the results until they were presented publicly. The manuscript may not reflect Pfizer’s opinion or beliefs. Further, the results and conclusions are those of the authors and no official endorsement by Manitoba Health was intended or should be inferred.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Kozyrskyj, A., Raymond, C. & Racher, A. Characterizing early prescribers of newly marketed drugs in Canada: a population-based study. Eur J Clin Pharmacol 63, 597–604 (2007). https://doi.org/10.1007/s00228-007-0277-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00228-007-0277-5