Abstract
Purpose
This study investigated the effect of the herbal medicine baicalin on bupropion hydroxylation, a probe reaction for CYP2B6 activity related to different CYP2B6 genotype groups.
Method
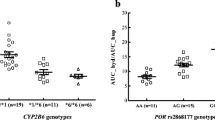
Seventeen healthy male volunteers (6 CYP2B6*1/*1, 6 CYP2B6*1/*6, and 5 CYP2B6*6/*6) received orally administered bupropion alone and during daily treatment with baicalin. Blood samples were taken up to 72 h after each bupropion dose, and pharmacokinetics profiles were determined on days 1 and 25 for bupropion and hydroxybupropion.
Result
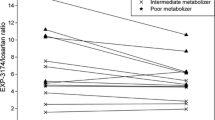
Baicalin administration increased hydroxybupropion maximum plasma concentration (C max) by 73% [90% confidence interval (CI), 44–108%; P < 0.01] and the area under the concentration time curve extrapolated to infinity (AUC0–∞) of hydroxybupropion by 87% (90% CI, 48–137%; P < 0.01), with no change in the elimination half-life of hydroxybupropion. Baicalin increased the AUC0–∞ ratio of hydroxybupropion to bupropion by 63% (90% CI, 38–92%; P < 0.01).
Conclusion
Baicalin significantly induced CYP2B6-catalyzed bupropion hydroxylation, and the effects of baicalin on other CYP2B6 substrate drugs deserve further investigation.



Similar content being viewed by others
References
Lu T (2007) Comparative pharmacokinetics of baicalin after oral administration of pure baicalin, Radix scutellariae extract and Huang-Lian-Jie-Du-Tang to rats. J Ethnopharmacol 110:412–418
Kawashima K, Fujimura Y, Makino T, Kano Y (2006) Pharmacological properties of traditional medicine (XXXII): protective effects of hangeshashinto and the combinations of its major constituents on gastric lesions in rats. Biol Pharm Bull 29:1973–1975
Li CY, Chiu CH, Huang HS, Lin CH, Wu TS (2006) High-performance liquid chromatographic method for simultaneous quantification of eight major biologically active ingredients in ‘Da-Chai-Hu-Tang’ preparation. Biomed Chromatogr 20:305–308
Lo YC et al (2005) San-Huang-Xie-Xin-Tang attenuates inflammatory responses in lipopolysaccharide-exposed rat lungs. J Ethnopharmacol 101:68–74
Liu CT, Wu CY, Weng YM, Tseng CY (2005) Ultrasound-assisted extraction methodology as a tool to improve the antioxidant properties of herbal drug Xiao-chia-hu-tang. J Ethnopharmacol 99:293–300
Zuo F et al (2003) Pharmacokinetic study on the multi-constituents of Huangqin-Tang decoction in rats. Biol Pharm Bull 26:911–919
Lininger SW, Gaby AR, Austin S, Brown DJ, Wright JV, Duncan A (2000) The natural pharmacy. Random House, New York
McGuffin M, Hobbs C, Upton R, Goldberg A (1997) American Herbal Products Association botanical safety handbook. CRC Press, Boca Raton
Hou YN, Zhu XY, Cheng GF (2000) Effects of baicalin on liver microsomal cytochrome P450 system. Acta Pharm Sin 35:890–892
Che QM, Huang XL, Li YM, Kun Z, Teruaki A, Masao H (2001) Studies on metabolites of baicalin in human urine (in Chinese). Zhongguo Zhong Yao Za Zhi 26(11):768–769
Fan L et al (2008) The effect of herbal medicine baicalin on pharmacokinetics of rosuvastatin, substrate of organic anion-transporting polypeptide 1B1. Clin Pharmacol Ther 83:471–476
Ekins S et al (1998) Further characterization of the expression in liver and catalytic activity of CYP2B6. J Pharmacol Exp Ther 286:1253–1259
Gervot L et al (1999) Human CYP2B6: expression, inducibility and catalytic activities. Pharmacogenetics 9:295–306
Nolan D, Phillips E, Mallal S (2006) Efavirenz and CYP2B6 polymorphism: implications for drug toxicity and resistance. Clin Infect Dis 42:408–410
Faucette SR et al (2004) Regulation of CYP2B6 in primary human hepatocytes by prototypical inducers. Drug Metab Dispos 32:348–358
Walsky RL, Astuccio AV, Obach RS (2006) Evaluation of 227 drugs for in vitro inhibition of cytochrome P450 2B6. J Clin Pharmacol 46:1426–1438
Turpeinen M, Raunio H, Pelkonen O (2006) The functional role of CYP2B6 in human drug metabolism: substrates and inhibitors in vitro, in vivo and in silico. Curr Drug Metab 7:705–714
Kirchheiner J et al (2003) Bupropion and 4-OH-bupropion pharmacokinetics in relation to genetic polymorphisms in CYP2B6. Pharmacogenetics 13:619–626
Jacob RM, Johnstone EC, Neville MJ, Walton RT (2004) Identification of CYP2B6 sequence variants by use of multiplex PCR with allele-specific genotyping. Clin Chem 50:1372–1377
Zukunft J et al (2005) A natural CYP2B6 TATA box polymorphism (-82T→C) leading to enhanced transcription and relocation of the transcriptional start site. Mol Pharmacol 67:1772–1782
Klein K et al (2005) Genetic variability of CYP2B6 in populations of African and Asian origin: allele frequencies, novel functional variants, and possible implications for anti-HIV therapy with efavirenz. Pharmacogenet Genomics 15:861–873
Guan S, Huang M, Chan E, Chen X, Duan W, Zhou SF (2006) Genetic polymorphisms of cytochrome P450 2B6 gene in Han Chinese. Eur J Pharm Sci 29:14–21
Cho JY et al (2004) Haplotype structure and allele frequencies of CYP2B6 in a Korean population. Drug Metab Dispos 32:1341–1344
Tsuchiya K et al (2004) Homozygous CYP2B6 *6 (Q172H and K262R) correlates with high plasma efavirenz concentrations in HIV-1 patients treated with standard efavirenz-containing regimens. Biochem Biophys Res Commun 319:1322–1326
Lee AM et al (2007) CYP2B6 genotype alters abstinence rates in a bupropion smoking cessation trial. Biol Psychiatry 62:635–641
Hesse LM et al (2000) CYP2B6 mediates the in vitro hydroxylation of bupropion: potential drug interactions with other antidepressants. Drug Metab Dispos 28:1176–1183
Faucette SR et al (2000) Validation of bupropion hydroxylation as a selective marker of human cytochrome P450 2B6 catalytic activity. Drug Metab Dispos 28:1222–1230
Faucette SR, Hawke RL, Shord SS, Lecluyse EL, Lindley CM (2001) Evaluation of the contribution of cytochrome P450 3A4 to human liver microsomal bupropion hydroxylation. Drug Metab Dispos 29:1123–1129
Dunner DL et al (1998) A prospective safety surveillance study for bupropion sustained-release in the treatment of depression. J Clin Psychiatry 59:366–373
Johnston JA et al (2001) Relationship between drug exposure and the efficacy and safety of bupropion sustained release for smoking cessation. Nicotine Tob Res 3:131–140
Bondarev ML, Bondareva TS, Young R, Glennon RA (2003) Behavioral and biochemical investigations of bupropion metabolites. Eur J Pharmacol 474:85–93
Damaj MI et al (2004) Enantioselective effects of hydroxy metabolites of bupropion on behavior and on function of monoamine transporters and nicotinic receptors. Mol Pharmacol 66:675–682
Palovaara S, Pelkonen O, Uusitalo J, Lundgren S, Laine K (2003) Inhibition of cytochrome P450 2B6 activity by hormone replacement therapy and oral contraceptive as measured by bupropion hydroxylation. Clin Pharmacol Ther 74:326–333
Turpeinen M et al (2005) Effect of clopidogrel and ticlopidine on cytochrome P450 2B6 activity as measured by bupropion hydroxylation. Clin Pharmacol Ther 77:553–559
Loboz KK et al (2006) Cytochrome P450 2B6 activity as measured by bupropion hydroxylation: effect of induction by rifampin and ethnicity. Clin Pharmacol Ther 80:75–84
Hogeland GW, Swindells S, McNabb JC, Kashuba AD, Yee GC, Lindley CM (2007) Lopinavir/ritonavir reduces bupropion plasma concentrations in healthy subjects. Clin Pharmacol Ther 81:69–75
Farid NA et al (2008) Prasugrel, a new thienopyridine antiplatelet drug, weakly inhibits cytochrome P450 2B6 in humans. J Clin Pharmacol 48:53–59
Hesse LM, Greenblatt DJ, Von Moltke LL, Court MH (2006) Ritonavir has minimal impact on the pharmacokinetic disposition of a single dose of bupropion administered to human volunteers. J Clin Pharmacol 46:567–576
Haynes KFS, Lindley C, LeCluyse E, Shord S, Hawke R (2002) Evaluation of buproprion hydroxylation as a probe for cytochrome P450 2B6 activity in cultured human hepatocytes. Poster. American College of Clinical Pharmacy Spring Practice and Research Forum, Savannah, GA, 10 April 2002
Borges V, Yang E, Dunn J, Henion J (2004) High-throughput liquid chromatography-tandem mass spectrometry determination of bupropion and its metabolites in human, mouse and rat plasma using a monolithic column. J Chromatogr B Analyt Technol Biomed Life Sci 25:277–287
Hesse LM et al (2004) Pharmacogenetic determinants of interindividual variability in bupropion hydroxylation by cytochrome P450 2B6 in human liver microsomes. Pharmacogenetics 14:225–238
Hesse LM, Sakai Y, Vishnuvardhan D, Li AP, Von Moltke LL, Greenblatt DJ (2003) Effect of bupropion on CYP2B6 and CYP3A4 catalytic activity, immunoreactive protein and mRNA levels in primary human hepatocytes: comparison with rifampicin. J Pharm Pharmacol 55:1229–1239
Turpeinen M, Nieminen R, Juntunen T, Taavitsainen P, Raunio H, Pelkonen O (2004) Selective inhibition of CYP2B6-catalyzed bupropion hydroxylation in human liver microsomes in vitro. Drug Metab Dispos 32:626–631
Stewart JJ et al (2001) Single-dose pharmacokinetics of bupropion in adolescents: effects of smoking status and gender. J Clin Pharmacol 41:770–778
Lai MY, Hsiu SL, Tsai SY, Hou YC, Chao PD (2003) Comparison of metabolic pharmacokinetics of baicalin and baicalein in rats. J Pharm Pharmacol 55(2):205–209
Taiming L, Xuehua J (2006) Investigation of the absorption mechanisms of baicalin and baicalein in rats. J Pharm Sci 95:1326–1333
Morris ME, Zhang S (2006) Flavonoid-drug interactions: effects of flavonoids on ABC transporters. Life Sci 78:2116–2130
Moon YJ, Wang X, Morris ME (2006) Dietary flavonoids: effects on xenobiotic and carcinogen metabolism. Toxicol In Vitro 20:187–210
Hong SS, Seo K, Lim SC, Han HK (2007) Interaction characteristics of flavonoids with human organic anion transporter 1 (hOAT1) and 3 (hOAT3). Pharmacol Res 56:468–473
Ekins S, Wrighton SA (1999) The role of CYP2B6 in human xenobiotic metabolism. Drug Metab Rev 31:719–754
Sridar C, Kent UM, Notley LM, Gillam EM, Hollenberg PF (2002) Effect of tamoxifen on the enzymatic activity of human cytochrome CYP2B6. J Pharmacol Exp Ther 301:945–952
Kharasch ED, Hoffer C, Whittington D, Sheffels P (2004) Role of hepatic and intestinal cytochrome CYP3A and CYP2B6 in the metabolism, disposition and miotic effects of methadone. Clin Pharmacol Ther 76:250–269
Totah RA, Allen KE, Sheffels P, Whittington D, Kharasch ED (2007) Enantiomeric metabolic interactions and stereoselective human methadone metabolism. J Pharmacol Exp Ther 321:389–399
Acknowledgements
This work was supported by research grants from the National Natural Science Foundation of China 30528026, 30300428, 30672497, 30500623, and 30572225, by the China Medical Board of New York grant 01-755, and the Hunan Health Research Foundation of Traditional Chinese Medicine grant 204041.
Conflict of interest
None of the authors has any conflict of interest regarding this study.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Fan, L., Wang, JC., Jiang, F. et al. Induction of cytochrome P450 2B6 activity by the herbal medicine baicalin as measured by bupropion hydroxylation. Eur J Clin Pharmacol 65, 403–409 (2009). https://doi.org/10.1007/s00228-008-0594-3
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00228-008-0594-3