Abstract
Purpose
The aim of this study was to describe suspected adverse reactions (ARs) associated with herbal products used for weight control in Italy.
Methods
Spontaneous reports of suspected ARs associated with herbal products used for weight control were collected by the Italian National Institute of Health (April 2002 to June 2010), and the causality assessment was performed.
Results
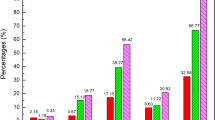
Forty-six of the suspected ARs were associated with herbal products used for weight control. Women were involved in 85% of the reports. The reactions affected mainly the cardiovascular system, the skin, the digestive system, the central nervous system, and the liver. A large proportion of ARs were serious. In more than half of the suspected ARs, the use of other therapies (herbs and/or drugs) was reported, while concomitant conditions were present in 22% of the reports.
Conclusions
The use of herbal dietary supplements for weight loss is associated with several ARs. Considering the risk/benefit ratio, consumers should pay attention when using these products.
Similar content being viewed by others
References
World Health Organization (2007) WHO European Ministerial Conference on Counteracting Obesity—Conference Report. Denmark, pp 1–36
Dietz B, Bolton JL (2007) Botanical dietary supplements gone bad. Chem Res Toxicol 20:586–590
Hasani-Ranjbar S, Nayebi N, Larijani B, Abdollahi M (2009) A systematic review of the efficacy and safety of herbal medicines used in the treatment of obesity. World J Gastroenterol 15:3073–3085
Lenz TL, Hamilton WR (2004) Supplemental products used for weight loss. J Am Pharm Assoc 44:59–67
Bray GA (2008) Are non-prescription medications needed for weight control? Obesity 16:509–714
Adachi M, Saito H, Kobayashi H, Horie Y, Kato S, Yoshioka M, Ishii H (2003) Hepatic injury in 12 patients taking the herbal weight loss AIDS Chaso or Onshido. Ann Intern Med 139:488–492
Ministero della salute. Alimenti particolari e integratori. http://www.salute.gov.it/alimentiParticolariIntegratori/paginaInternaMenuAlimentiParticolariIntegratori.jsp?id=1267&menu=integratori. Accessed 3 September 2010
Kennedy DA, Seely D (2010) Clinically based evidence of drug-herb interactions: a systematic review. Expert Opin Drug Saf 9:79–124
Tachjian A, Maria V, Jahangir A (2010) Use of herbal products and potential interactions in patients with cardiovascular diseases. J Am Coll Cardiol 55:515–525
Edwards IR, Aronson JK (2000) Adverse drug reactions: definitions, diagnosis, and management. Lancet 356:1255–1259
World Health Organization (WHO), Uppsala Monitoring Centre (2004) The use of the WHO-UMC system for standardized causality assessment. WHO [online]. http://www.who-umc.org/graphics/4409.pdf Accessed 3 September 2010
AIFA (2010) Aumentano le segnalazioni nel 2009. Reazioni – Bollettino di Farmacovigilanza 16:7–8
Medicines and Healthcare products Regulatory Agency (2008) Public health risk with herbal medicines: an overview. Marked Towers, London. http://www.mhra.gov.uk/Howweregulate/Medicines/Herbalmedicines/HerbalSafetyNews/index.htm Accessed 25 October 2010
Eurispes—Rapporto Italia 2010 (scheda 55) (2010) Curarsi con le medicine non convenzionali. http://www.infoculturale.it/Eurispes-RI2010_scheda%2055.pdf Accessed 26 October 2010
Barnes J (2003) Pharmacovigilance of herbal medicines. A UK perspective. Drug Saf 26:829–851
Ben-Arye E, Karkabi S, Shapira C, Schiff E, Lavie O, Keshet Y (2009) Complementary medicine in the primary care setting: results of a survey of gender and cultural patterns in Israel. Gend Med 26:384–397
Goh LY, Vitry AI, Semple SJ, Esterman A, Luszcz MA (2009) Self-medication with over-the-counter drugs and complementary medications in South Australia’s elderly population. BMC Complement Altern Med 9:42
Compton R, Spiller HA, Bosse GM (2005) Fatal fluoxetine ingestion with postmortem blood concentrations. Clin Toxicol (Phila) 43(4):277–279
Perrone J, Phillips C, Gaieski D (2008) Occult metformin toxicity in three patients with profound lactic acidosis. J Emerg Med. doi:10.1016/j.jemermed.2007.11.055
Holmes RO, Tavee J (2008) Vasospasm and stroke attributable to ephedra-free xenadrine: case report. Mil Med 173:708–710
Shord SS, Shah K, Lukose A (2009) Drug-botanical interactions: a review of the laboratory, animal, and human data for 8 common botanicals. Integr Cancer Ther 8:208–227
Ulbricht C, Chao W, Costa D, Rusie-Seamon E, Weissner W, Woods J (2008) Clinical evidence of herb-drug interactions: a systematic review by the natural standard research collaboration. Curr Drug Metab 9:1063–1120
Henderson S, Magu B, Rasmussen C, Lancaster S, Kerksick C, Smith P, Melton C, Cowan P, Greenwood M, Earnest C, Almada A, Milnor P, Magrans T, Bowden R, Ounpraseuth S, Thomas A, Kreider RB (2005) Effects of Coleus forskohlii supplementation on body composition and hematological profiles in mildly overweight women. J Int Soc Sports Nutr 2:54–62
Restani P, Marangon K, Colombo ML (2006) Problemi di controllo qualità e sicurezza di integratori alimentari e preparazioni magistrali contenti estratti di Coleus forskholii. XIV Congresso Nazionale della Società Italiana di Tossicologia. Istituto Superiore di Sanità, ISTISAN, Roma, p 59
Menniti-Ippolito F, Mazzanti G, Santuccio C, Moro PA, Calapai G, Firenzuoli F, Valeri A, Raschetti R (2008) Surveillance of suspected adverse reactions to natural health products in Italy. Pharmacoepidemiol Drug Saf 17:626–635
Koch E, Jaggy H, Chatterjee SS (2000) Evidence for immunotoxic effects of crude Ginkgo biloba L. leaf extracts using the popliteal lymph node assay in the mouse. Int J Immunopharmacol 22:229–236
Jacobsson I, Jönsson AK, Gerdén B, Hägg S (2009) Spontaneously reported adverse reactions in association with complementary and alternative medicine substances in Sweden. Pharmacoepidemiol Drug Saf 18:1039–1047
Chiu AE, Lane AT, Kimball AB (2002) Diffuse morbilliform eruption after consumption of ginkgo biloba supplement. J Am Acad Dermatol 46:145–146
Schatzki R, Je G (1953) Dysphagia due to a diaphragm-like localized narrowing in the lower esophagus (lower esophageal ring). J Am J Roentgenol Radium Ther Nucl Med 70:911–922
Miller S, CJr H, Ochsner JL (1988) Spontaneous perforation of the esophagus associated with a lower esophageal ring. Am J Gastroenterol 83:1405–1408
Lewis JH (1992) Esophageal and small bowel obstruction from guar gum-containing “diet pills”: analysis of 26 cases reported to the Food and Drug Administration. Am J Gastroenterol 87:1424–1428
Mazzanti G, Menniti-Ippolito F, Moro PA, Cassetti F, Raschetti R, Santuccio C, Mastrangelo S (2009) Hepatotoxicity from green tea: a review of the literature and two unpublished cases. Eur J Clin Pharmacol 65:331–341
Ramos R, Mascarenhas J, Duarte P, Vicente C, Casteleiro C (2009) Conjugated linoleic acid-induced toxic hepatitis: first case report. Dig Dis Sci 54:1141–1143
Actis GC, Bugianesi E, Ottobrelli A, Rizzetto M (2007) Fatal liver failure following food supplements during chronic treatment with montelukast. Dig Liver Dis 39:953–955
Walsky RL, Obach RS, Gaman EA, Gleeson JP, Proctor WR (2005) Selective inhibition of human cytochrome P4502C8 by montelukast. Drug Metab Dispos 33(3):413–418
Saito M, Hirata-Koizumi M, Matsumoto M, Urano T, Hasegawa R (2005) Undesirable effects of citrus juice on the pharmacokinetics of drugs: focus on recent studies. Drug Saf 28(8):677–694
Shilo S, Hirsch HJ (1986) Iodine-induced hyperthyroidism in a patient with a normal thyroid gland. Postgrad Med J 62:661–662
Eliason BC (1998) Transient hyperthyroidism in a patient taking dietary supplements containing kelp. J Am Board Fam Pract 11:478–480
Müssig K, Thamer C, Bares R, Lipp HP, Häring HU, Gallwitz B (2006) Iodine-induced thyrotoxicosis after ingestion of kelp-containing tea. J Gen Intern Med 21:C11–C14
Sharpe PA, Granner ML, Conway JM, Ainsworth BE, Dobre M (2006) Availability of weight-loss supplements: results of an audit of retail outlets in a southeastern city. J Am Diet Assoc 106(12):2045–2051
World Health Organization (2002) The importance of pharmacovigilance: safety monitoring of medicinal products. http://www.who.int/medicines/areas/quality_safety/safety_efficacy/pharmvigi/en/index.html. Accessed 3 September 2010
Conflict of interest statement
The authors declare that there are no conflicts of interest.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Vitalone, A., Menniti-Ippolito, F., Moro, P.A. et al. Suspected adverse reactions associated with herbal products used for weight loss: a case series reported to the Italian National Institute of Health. Eur J Clin Pharmacol 67, 215–224 (2011). https://doi.org/10.1007/s00228-010-0981-4
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00228-010-0981-4