Abstract
Purpose
To evaluate the quality of data reporting and statistical methods performed in drug utilization studies in the pediatric population.
Methods
Drug utilization studies evaluating all drug prescriptions to children and adolescents published between January 1994 and December 2011 were retrieved and analyzed. For each study, information on measures of exposure/consumption, the covariates considered, descriptive and inferential analyses, statistical tests, and methods of data reporting was extracted. An overall quality score was created for each study using a 12-item checklist that took into account the presence of outcome measures, covariates of measures, descriptive measures, statistical tests, and graphical representation.
Results
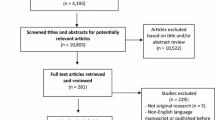
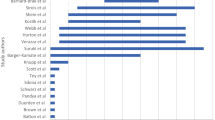
A total of 22 studies were reviewed and analyzed. Of these, 20 studies reported at least one descriptive measure. The mean was the most commonly used measure (18 studies), but only five of these also reported the standard deviation. Statistical analyses were performed in 12 studies, with the chi-square test being the most commonly performed test. Graphs were presented in 14 papers. Sixteen papers reported the number of drug prescriptions and/or packages, and ten reported the prevalence of the drug prescription. The mean quality score was 8 (median 9). Only seven of the 22 studies received a score of ≥10, while four studies received a score of <6.
Conclusions
Our findings document that only a few of the studies reviewed applied statistical methods and reported data in a satisfactory manner. We therefore conclude that the methodology of drug utilization studies needs to be improved.
Similar content being viewed by others
References
Strom BL (ed) (2005) Pharmacoepidemiology. John Wiley & Sons, West Sussex
MacLeod SM (1991) Pharmacoepidemiology: a health imperative. J Clin Epidemiol 44(12):1285–1286
Boots I, Sukhai RN, Klein RH, Holl RA, Wit JM, Cohen AF, Burggraaf J (2007) Stimulation programs for pediatric drug research–do children really benefit? Eur J Pediatr 166(8):849–855
Hoppu K (2008) Paediatric clinical pharmacology: at the beginning of a new era. Eur J Clin Pharmacol 64(2):201–205
Olski TM, Lampus SF, Gherarducci G, Saint RA (2011) Three years of paediatric regulation in the European Union. Eur J Clin Pharmacol 67(3):245–252
Hoppu K, Anabwani G, Garcia-Bournissen F, Gazarian M, Kearns GL, Nakamura H, Peterson RG, Sri RS, de Wildt SN (2012) The status of paediatric medicines initiatives around the world-what has happened and what has not? Eur J Clin Pharmacol 68(1):1–10
Clavenna A, Bonati M (2009) Drug prescriptions to outpatient children: a review of the literature. Eur J Clin Pharmacol 65(8):749–755
Bianchi M, Clavenna A, Bonati M (2010) Inter-country variations in anti-asthmatic drug prescriptions for children. Systematic review of studies published during the 2000-2009 period. Eur J Clin Pharmacol 66(9):929–936
Clavenna A, Bonati M (2007) Antidepressant prescriptions in paediatric outpatients in Europe. Paediatr Perinat Drug Ther 8(3):103–108
Rossignoli A, Clavenna A, Bonati M (2007) Antibiotic prescription and prevalence rate in the outpatient paediatric population: analysis of surveys published during 2000–2005. Eur J Clin Pharmacol 63(12):1099–1106
Verhamme K, Sturkenboom M (2011) Study designs in paediatric pharmacoepidemiology. Eur J Clin Pharmacol 67(Suppl 1):67–74
WHO Collaborating Centre for Drug Statistics Methodology (2009) Guidelines for ATC classification and DDD assignment 2010. Available at: http://www.whocc.no/filearchive/publications/2010guidelines.pdf. Accessed 2 Dec 2010
Hahn GH, Koch A, Melbye M, Molbak K (2005) Pattern of drug prescription for children under the age of four years in a population in Greenland. Acta Paediatr 94(1):99–106
Straand J, Rokstad K, Heggedal U (1998) Drug prescribing for children in general practice. A report from the More & Romsdal Prescription Study. Acta Paediatr 87(2):218–224
Sanz E, Hernandez MA, Ratchina S, Stratchounsky L, Peire MA, Lapeyre-Mestre M, Horen B, Kriska M, Krajnakova H, Momcheva H, Encheva D, Martinez-Mir I, Palop V (2004) Drug utilisation in outpatient children. A comparison among Tenerife, Valencia, and Barcelona (Spain), Toulouse (France), Sofia (Bulgaria), Bratislava (Slovakia) and Smolensk (Russia). Eur J Clin Pharmacol 60(2):127–134
Niclasen BV, Moller SM, Christensen RB (1995) Drug prescription to children living in the Arctic. An investigation from Nuuk, Greenland. Arctic Med Res 54[Suppl 1]:95–100
Thrane N, Sorensen HT (1999) A one-year population-based study of drug prescriptions for Danish children. Acta Paediatr 88(10):1131–1136
Schirm E, van den Berg P, Gebben H, Sauer P, de Jong-van den Berg L (2000) Drug use of children in the community assessed through pharmacy dispensing data. Br J Clin Pharmacol 50(5):473–478
Madsen H, Andersen M, Hallas J (2001) Drug prescribing among Danish children: a population-based study. Eur J Clin Pharmacol 57(2):159–165
Cazzato T, Pandolfini C, Campi R, Bonati M (2001) Drug prescribing in out-patient children in Southern Italy. Eur J Clin Pharmacol 57(8):611–616
Hall J, Martin I (2003) Prescribing for teenagers in New Zealand general practice. N Z Med J 116(1186):U685
Sturkenboom MC, Verhamme KM, Nicolosi A, Murray ML, Neubert A, Caudri D, Picelli G, Sen EF, Giaquinto C, Cantarutti L, Baiardi P, Felisi MG, Ceci A, Wong IC (2008) Drug use in children: cohort study in three European countries. Br Med J 337:a2245
Nwolisa CE, Erinaugha EU, Ofoleta SI (2006) Prescribing practices of doctors attending to under fives in a children’s outpatient clinic in Owerri, Nigeria. J Trop Pediatr 52(3):197–200
Al Khaja KA, Al Ansari TM, Damanhori AH, Sequeira RP (2007) Evaluation of drug utilization and prescribing errors in infants: a primary care prescription-based study. Health Policy 81(2–3):350–357
Nizami SQ, Khan IA, Bhutta ZA (1997) Paediatric prescribing in Karachi. J Pak Med Assoc 47(1):29–32
Phillips-Howard PA, Wannemuehler KA, ter Kuile FO, Hawley WA, Kolczak MS, Odhacha A, Vulule JM, Nahlen BL (2003) Diagnostic and prescribing practices in peripheral health facilities in rural western Kenya. Am J Trop Med Hyg 68(4 Suppl):44–49
Hong SH, Shepherd MD (1996) Outpatient prescription drug use by children enrolled in five drug benefit plans. Clin Ther 18(3):528–545
Niclasen BV (2006) Changes in drug prescription over a decade in an Arctic child population. Acta Paediatr 95(11):1456–1460
Clavenna A, Sequi M, Bortolotti A, Merlino L, Fortino I, Bonati M (2009) Determinants of the drug utilization profile in the paediatric population in Italy’s Lombardy Region. Br J Clin Pharmacol 67(5):565–571
Clavenna A, Berti A, Gualandi L, Rossi E, De Rosa M, Bonati M (2009) Drug utilisation profile in the Italian paediatric population. Eur J Pediatr 168(2):173–180
Zaki A, Abdel-Fattah M, Bassili A, Arafa M, Bedwani R (1999) The use of medication in infants in Alexandria, Egypt. East Mediterr Health J 5(2):320–327
Hawkins N, Golding J (1995) A survey of the administration of drugs to young infants. Br J Clin Pharmacol 40(1):79–82
Stoelben S, Krappweis J, Rossler G, Kirch W (2000) Adolescents’ drug use and drug knowledge. Eur J Pediatr 159(8):608–614
Nsimba SE (2007) Assessing the performance, practices and roles of drug sellers/dispensers and mothers’/guardians’ behaviour for common childhood conditions in Kibaha district, Tanzania. Trop Dr 37(4):197–201
International Society for Pharmacoepidemiology (2008) Guidelines for good pharmacoepidemiology practices (GPP). Pharmacoepidemiol Drug Saf 17(2):200–208
Acknowledgments
The authors would like to thank Dr. Filomena Fortinguerra and Dr. Daniele Piovani for their assistance in the quality evaluation of the studies.
Conflicts of interest
None.
Author information
Authors and Affiliations
Corresponding author
Appendix 1. Quality checklist (Yes = 1; No = 0)
Appendix 1. Quality checklist (Yes = 1; No = 0)
-
a)
Outcome measures:
-
1.
Is prevalence reported?
-
2.
Is drug consumption (number of prescriptions, number of medication packages) reported?
-
1.
-
b)
Covariates:
-
3.
Were the outcome measures stratified by age?
-
4.
Were the outcome measures stratified by gender?
-
3.
-
c)
Drug:
-
5.
Did the analyses consider all the drugs (including OTC and nonreimbursable drugs)?
-
6.
Were the outcome measures stratified by drug or drug class?
-
5.
-
d)
Descriptive analyses:
-
7.
Did the study report at least one measure of position (e.g,. mean, median)?
-
8.
Did the study report at least one measure of dispersion (e.g., SD, range, 95 % CI)?
-
7.
-
e)
Inferential analyses/tests:
-
9.
Were statistical tests performed?
-
10.
Were the analyses adjusted by age and/or gender?
-
9.
-
f)
Data reporting:
-
11.
Did the paper report tables?
-
12.
Did the paper report graphs?
-
11.
Rights and permissions
About this article
Cite this article
Sequi, M., Campi, R., Clavenna, A. et al. Methods in pharmacoepidemiology: a review of statistical analyses and data reporting in pediatric drug utilization studies. Eur J Clin Pharmacol 69, 599–604 (2013). https://doi.org/10.1007/s00228-012-1354-y
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00228-012-1354-y