Abstract
Purpose
Citalopram is a selective serotonin reuptake inhibitor (SSRI) antidepressant that is widely used in clinical practice. Recent data have indicated that high therapeutic citalopram doses may cause electrocardiographic abnormalities, and the regulatory authorities have amended its licenced dosage. The present manuscript reviews the available data concerning citalopram and cardiac toxicity.
Methods
Published data concerning the cardiac effects of citalopram were ascertained, and clinical data were considered separately between adverse effects arising from therapeutic use versus toxicity in the setting of intentional overdose.
Results
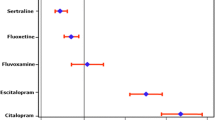
The occurrence of electrocardiographic abnormalities has long been recognised as a complication of acute citalopram overdose; a dose-effect relationship for QT prolongation has been described in a number of large case series, including several cases of torsades de pointes. In contrast, few data indicate the occurrence of QT prolongation and arrhythmia after therapeutic doses, and a dose-effect relationship within the therapeutic range has only recently been established. Citalopram is more likely to cause QT prolongation in patients with metabolic disturbance or pre-existing cardiac disease.
Conclusions
A dose-effect relationship for QT prolongation exists across a broad range of citalopram doses, such that caution must be exercised when prescribing high doses or if there are co-existent risk factors for QT effects. The available data illustrate how clinical toxicity data may offer an earlier signal of cardiac effects than ascertained from conventional pharmacovigilance methods.

Similar content being viewed by others
References
US Food and Drug Administration (2012) Abnormal heart rhythms associated with high doses of celexa (citalopram hydrobromide). Available from: http://www.fda.gov/Safety/MedWatch/SafetyInformation/SafetyAlertsforHumanMedicalProducts/ucm269481.htm (accessed 12/06/2012)
US Food and Drug Administration (2012) Celexa (citalopram hydrobromide) drug safety communication: revised recommendations, potential risk of abnormal heart rhythms. Available from: http://www.fda.gov/Safety/MedWatch/SafetyInformation/SafetyAlertsforHumanMedicalProducts/ucm297624.htm (accessed 12/06/2012)
Medicines and Healthcare products Regulatory Agency (2011) Citalopram and escitalopram: QT interval prolongation: new maximum daily dose restrictions (including in elderly patients), contraindications, and warnings information. Available from: http://www.mhra.gov.uk/Safetyinformation/DrugSafetyUpdate/CON137769 (accessed 12/06/2012)
Pollock BG (2001) Citalopram: a comprehensive review. Expert Opin Pharmacother 2:681–698
Le Bloc’h Y, Woggon B, Weissenrieder H, Brawand-Amey M, Spagnoli J, Eap CB, Baumann P (2003) Routine therapeutic drug monitoring in patients treated with 10–360 mg/day citalopram. Ther Drug Monit 25:600–608
Mitcheson JS (2003) Drug binding to HERG channels: evidence for a “non-aromatic” binding site for fluvoxamine. Br J Pharmacol 139:883–884
Witchel HJ, Pabbathi VK, Hofmann G, Paul AA, Hancox JC (2002) Inhibitory actions of the selective serontonin re-uptake inhibitor citalopram on HERG and ventricular L-type calcium currents. FEBS Lett 512:59–66
Fredricson Overø K (1982) Kinetics of citalopram in test animals; drug exposure in safety studies. Prog Neuropsychopharmacol Biol Psychiatry 6:297–309
Boeck V, Overø KF, Svendsen O (1982) Studies on acute toxicity and drug levels of citalopram in the dog. Acta Pharmacol Toxicol 50:169–174
Muldoon C (1996) The safety and tolerability of citalopram. Int Clin Psychopharmacol 11(Suppl 1):35–40
Rasmussen SL, Overo KF, Tanghoj P (1999) Cardiac safety of citalopram: prospective trials and retrospective analyses. J Clin Psychopharmacol 19:407–415
Kanjanauthai S, Kanluen T, Chareonthaitawee P (2008) Citalopram induced torsades de pointes, a rare life threatening side effect. Int J Cardiol 131:e33–e34
Favre MP, Sztajzel J, Berschy G (1999) Bradycardia during citalopram treatment: a case report. Pharmacol Res 57:149–150
Deshmukh A, Ulveling K, Alla V, Abuissa H, Airey K (2012) Prolonged QTc interval and torsades de pointes induced by citalopram. Tex Heart Inst J 39:68–70
Tseng PT, Lee Y, Lin YE, Lin PY (2012) Low-dose escitalopram for 2 days associated with corrected QT interval prolongation in a middle-aged woman: a case report and literature review. Gen Hosp Psychiatry 34:210
Sala M, Vicentini A, Brambilla P, Montomoli C, Jogia JR, Caverzasi E, Bonzano A, Piccinelli M, Barale F, De Ferrari GM (2005) QT interval prolongation related to psychoactive drug treatment: a comparison of monotherapy versus polytherapy. Ann Gen Psychiatry 4:1
Isbister GK, Prior FH, Foy A (2001) Citalopram-induced bradycardia and presyncope. Ann Pharmacother 35:1552–1555
Slavícek J, Paclt I, Hamplová J, Kittnar O, Trefný Z, Horácek BM (1998) Antidepressant drugs and heart electrical field. Physiol Res 47:297–300
Aström-Lilja C, Odeberg JM, Ekman E, Hägg S (2008) Drug-induced torsades de pointes: a review of the Swedish pharmacovigilance database. Pharmacoepidemiol Drug Saf 17:587–592
Medicines and Healthcare products Regulatory Agency (2012) Citalopram drug analysis print. Available from: http://www.mhra.gov.uk/home/groups/public/documents/sentineldocuments/dap_1335337465722.pdf (accessed 12/06/2012)
Montgomery SA (1995) Selecting the optimum therapeutic dose of serotonin reuptake inhibitors: studies with citalopram. Int Clin Psychopharmacol 10(Suppl 1):23–27
Jimmink A, Caminada K, Hunfeld NG, Touw DJ (2008) Clinical toxicology of citalopram after acute intoxication with the sole drug or in combination with other drugs: overview of 26 cases. Ther Drug Monit 30:365–371
Catalano G, Catalano MC, Epstein MA, Tsambiras PE (2001) QTc interval prolongation associated with citalopram overdose: a case report literature review. Clin J Neuropharmacol 24:158–162
Tarabar AF, Hoffman RS, Lelson LS (2008) Citalopram overdose: late presentation if torsdae de pointes with cardiac arrest. J Med Toxicol 4:101–105
Grundemar L, Wohlfart B, Lagerstedt C, Bengtsson F, Eklundh G (1997) Symptoms and signs of severe citalopram overdose. Lancet 349:1602
Personne M, SjÖberg G, Persson H (1997) Citalopram overdose: review of cases treated in Swedish hospitals. J Toxicol Clin Toxicol 35:237–240
Rothenhäusler HB, Hoberl C, Ehrentrout S, Kapfhammer HP, Weber MM (2000) Suicide attempt by pure citalopram overdose causing long-lasting severe sinus bradycardia, hypotension and syncopes: successful therapy with a temporary pacemaker. Pharmacopsychiatry 33:150–152
Engebretsen KM, Harris CR, Wood JE (2003) Cardiotoxicity and late onset seizures with citalopram overdose. J Emerg Med 25:163–166
Chan CY, Waring WS (2007) Images in cardiovascular medicine. Tricyclic cardiotoxicity treated with sodium bicarbonate. Circulation 115:e63–e64
Liotier J, Coudoré F (2011) Drug monitoring of a case of citalopram overdosage. Drug Chem Toxicol 34:420–423
Masullo LN, Miller MA, Baker SD, Bose S, Levsky M (2006) Clinical course and toxicokinetic data following isolated citalopram overdose in an infant. Clin Toxicol 44:165–168
Kelly CA, Dhaun N, Laing WJ, Strachan FE, Good AM, Bateman DN (2004) Comparative toxicity of citalopram and the newer antidepressants after overdose. J Toxicol Clin Toxicol 42:67–71
Isbister GK, Bowe SJ, Dawson A, Whyte IM (2004) Relative toxicity of selective serotonin reuptake inhibitors (SSRIs) in overdose. J Toxicol Clin Toxicol 42:277–285
Friberg LE, Isbister GK, Duffull SB (2006) Pharmacokinetic-pharmacodynamic modelling of QT interval prolongation following citalopram overdoses. Br J Clin Pharmacol 61:177–190
Waring WS, Graham A, Gray J, Wilson AD, Howell C, Bateman DN (2010) Evaluation of a QT nomogram for risk assessment after antidepressant overdose. Br J Clin Pharmacol 70:881–885
de Gregorio C, Morabito G, Cerrito M, Dattilo G, Oreto G (2011) Citalopram induced long QT syndrome and torsade de pointes: role for concomitant therapy and disease. Int J Cardiol 148:226–228
Priskorn M, Larsen F, Segonzac A, Moulin M (1997) Pharmacokinetic interaction study of citalopram and cimetidine in healthy subjects. Eur J Clin Pharmacol 52:241–242
Wall AJ, Bateman DN, Waring WS (2009) Variability in the quality of overdose advice in Summary of Product Characteristics (SPC) documents: gut decontamination recommendations for CNS drugs. Br J Clin Pharmacol 67:83–87
Waring WS, McGettigan P (2011) Clinical toxicology and drug regulation: a United Kingdom perspective. Clin Toxicol 49:452–456
Hawton K, Bergen H, Waters K, Murphy E, Cooper J, Kapur N (2011) Impact of withdrawal of the analgesic Co-proxamol on nonfatal self-poisoning in the UK. Crisis 32:81–87
Conflict of interest statement
The authors declare that they have no conflict of interest.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Cooke, M.J., Waring, W.S. Citalopram and cardiac toxicity. Eur J Clin Pharmacol 69, 755–760 (2013). https://doi.org/10.1007/s00228-012-1408-1
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00228-012-1408-1