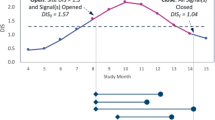
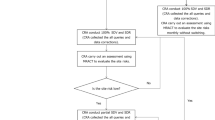
Abstract
Purpose
Monitoring is a costly requirement when conducting clinical trials. New regulatory guidance encourages the industry to consider alternative monitoring methods to the traditional 100 % source data verification (SDV) approach. The purpose of this literature review is to provide an overview of publications on different monitoring methods and their impact on subject safety data, data integrity, and monitoring cost.
Methods
The literature search was performed by keyword searches in MEDLINE and hand search of key journals. All publications were reviewed for details on how a monitoring approach impacted subject safety data, data integrity, or monitoring costs.
Results
Twenty-two publications were identified. Three publications showed that SDV has some value for detection of not initially reported adverse events and centralized statistical monitoring (CSM) captures atypical trends. Fourteen publications showed little objective evidence of improved data integrity with traditional monitoring such as 100 % SDV and sponsor queries as compared to reduced SDV, CSM, and remote monitoring. Eight publications proposed a potential for significant cost reductions of monitoring by reducing SDV without compromising the validity of the trial results.
Conclusions
One hundred percent SDV is not a rational method of ensuring data integrity and subject safety based on the high cost, and this literature review indicates that reduced SDV is a viable monitoring method. Alternative methods of monitoring such as centralized monitoring utilizing statistical tests are promising alternatives but have limitations as stand-alone tools. Reduced SDV combined with a centralized, risk-based approach may be the ideal solution to reduce monitoring costs while improving essential data quality.

Similar content being viewed by others
References
Food and Drug Administration (2011) 21 CFR part 312, subpart D: http://www.accessdata.fda.gov/scripts/cdrh/cfdocs/cfcfr/CFRSearch.cfm?CFRPart=312&showFR=1&subpartNode=21:5.0.1.1.3.4. and 21 CFR part 812, subpart C: https://www.accessdata.fda.gov/scripts/cdrh/cfdocs/cfcfr/CFRSearch.cfm?CFRPart=812&showFR=1&subpartNode=21:8.0.1.1.9.3
The International Conference on Harmonisation (1996) Guideline for good clinical practice: E6: http://www.ich.org/fileadmin/Public_Web_Site/ICH_Products/Guidelines/Efficacy/E6/E6_R1_Guideline.pdf
Food and Drug Administration (1988) Guidance for industry: guideline for the monitoring of clinical investigations: http://www.ahc.umn.edu/img/assets/19826/Clinical%20monitoring.pdf
Funning S, Grahnén A, Eriksson K, Kettis-Linblad Å (2009) Quality assurance within the scope of Good Clinical Practice (GCP)—what is the cost of GCP-related activities? A survey within the Swedish Association of the Pharmaceutical Industry (LIF)’s members. Qual Assur J 12(1):3–7
Scannell JW, Blanckley A, Boldon H, Warrington B (2012) Diagnosing the decline in pharmaceutical R&D efficiency. Nat Rev Drug Discov 11(3):191–200. doi:10.1038/nrd3681
Glickman SW, McHutchison JG, Peterson ED, Cairns CB, Harrington RA, Califf RM, Schulman KA (2009) Ethical and scientific implications of the globalization of clinical research. N Engl J Med 360(8):816–823. doi:10.1056/NEJMsb0803929
European Medicines Agency (2014) Reflection paper on risk based quality management in clinical trials: http://www.ema.europa.eu/docs/en_GB/document_library/Scientific_guideline/2013/11/WC500155491.pdf
Food and Drug Administration (2014) Guidance for industry, oversight of clinical investigations—a risk based approach to monitoring: http://www.fda.gov/downloads/Drugs/…/Guidances/UCM269919.pdf
Sheetz N, Wilson B, Benedict J, Huffman E, Lawton A, Travers M, Nadolny P, Young S, Given K, Florin L (2014) Evaluating source data verification as a quality control measure in clinical trials. Ther Innov Regul Sci. doi:10.1177/2168479014554400
Edwards P, Shakur H, Barnetson L, Prieto D, Evans S, Roberts I (2014) Central and statistical data monitoring in the Clinical Randomisation of an Antifibrinolytic in Significant Haemorrhage (CRASH-2) trial. Clin Trials 11(3):336–343. doi:10.1177/1740774513514145
Desmet L, Venet D, Doffagne E, Timmermans C, Burzykowski T, Legrand C, Buyse M (2014) Linear mixed-effects models for central statistical monitoring of multicenter clinical trials. Stat Med 33(30):5265–5279. doi:10.1002/sim.6294
Andersen JR, Byrjalsen I, Bihlet A, Kalakou F, Hoeck HC, Hansen G, Hansen HB, Karsdal MA, Riis BJ (2015) Impact of source data verification on data quality in clinical trials: an empirical post hoc analysis of three phase 3 randomized clinical trials. Br J Clin Pharmacol 79(4):660–668. doi:10.1111/bcp.12531
Tudur Smith C, Stocken DD, Dunn J, Cox T, Ghaneh P, Cunningham D, Neoptolemos JP (2012) The value of source data verification in a cancer clinical trial. PLoS One 7(12), e51623. doi:10.1371/journal.pone.0051623
Mitchel JT, Kim YJ, Choi J, Park G, Cappi S, Horn D, Kist M, D. Agostino RB Jr (2011) Evaluation of data entry errors and data changes to an electronic data capture clinical trial database. Drug Inf J 45(4):421–430. doi:10.1177/009286151104500404
Mitchel J, Kim Y, Hamrell M, Carrara D, Markowitz J, Cho T, Nora S, Gittleman D, Choi J (2014) Time to change the clinical trial monitoring paradigm. Appl Clin Trials
TransCelerate (2013) Position paper: risk-based monitoring methodology. http://www.transceleratebiopharmainc.com/wp-content/uploads/2013/10/TransCelerate-RBM-Position-Paper-FINAL-30MAY2013.pdf
Lienard JL, Quinaux E, Fabre-Guillevin E, Piedbois P, Jouhaud A, Decoster G, Buyse M, European Association for Research in O (2006) Impact of on-site initiation visits on patient recruitment and data quality in a randomized trial of adjuvant chemotherapy for breast cancer. Clin Trials 3(5):486–492. doi:10.1177/1740774506070807
Journot V, Perusat-Villetorte S, Bouyssou C, Couffin-Cadiergues S, Tall A, Chene G, Optimon Collaborative G (2013) Remote preenrollment checking of consent forms to reduce nonconformity. Clin Trials 10(3):449–459. doi:10.1177/1740774513480003
Bakobaki JM, Rauchenberger M, Joffe N, McCormack S, Stenning S, Meredith S (2012) The potential for central monitoring techniques to replace on-site monitoring: findings from an international multi-centre clinical trial. Clin Trials 9(2):257–264. doi:10.1177/1740774511427325
Tantsyura V, Dunn IM, Fendt K, Kim YJ, Waters J, Mitchel J (2015) Risk-based monitoring: a closer statistical look at source document verification, queries, study size effects, and data quality. Ther Innov Regul Sci. doi:10.1177/2168479015586001
Lindblad AS, Manukyan Z, Purohit-Sheth T, Gensler G, Okwesili P, Meeker-O’Connell A, Ball L, Marler JR (2014) Central site monitoring: results from a test of accuracy in identifying trials and sites failing Food and Drug Administration inspection. Clin Trials 11(2):205–217. doi:10.1177/1740774513508028
Kirkwood AA, Cox T, Hackshaw A (2013) Application of methods for central statistical monitoring in clinical trials. Clin Trials 10(5):783–806. doi:10.1177/1740774513494504
Venet D, Doffagne E, Burzykowski T, Beckers F, Tellier Y, Genevois-Marlin E, Becker U, Bee V, Wilson V, Legrand C, Buyse M (2012) A statistical approach to central monitoring of data quality in clinical trials. Clin Trials 9(6):705–713. doi:10.1177/1740774512447898
Nielsen E, Hyder D, Deng C (2014) A data-driven approach to risk-based source data verification. Ther Innov Regul Sci 48(2):173–180. doi:10.1177/2168479013496245
Tantsyura V, Dunn IM, Waters J, Fendt K, Kim YJ, Viola D, Mitchel J (2015) Extended risk-based monitoring model, on-demand query-driven source data verification, and their economic impact on clinical trial operations. Ther Innov Regul Sci. doi:10.1177/2168479015596020
Eisenstein EL, Collins R, Cracknell BS, Podesta O, Reid ED, Sandercock P, Shakhov Y, Terrin ML, Sellers MA, Califf RM, Granger CB, Diaz R (2008) Sensible approaches for reducing clinical trial costs. Clin Trials 5(1):75–84. doi:10.1177/1740774507087551
Pronker E, Geerts BF, Cohen A, Pieterse H (2011) Improving the quality of drug research or simply increasing its cost? An evidence-based study of the cost for data monitoring in clinical trials. Br J Clin Pharmacol 71(3):467–470. doi:10.1111/j.1365-2125.2010.03839.x
Mealer M, Kittelson J, Thompson BT, Wheeler AP, Magee JC, Sokol RJ, Moss M, Kahn MG (2013) Remote source document verification in two national clinical trials networks: a pilot study. PLoS One 8(12), e81890. doi:10.1371/journal.pone.0081890
Uren SC, Kirkman MB, Dalton BS, Zalcberg JR (2013) Reducing clinical trial monitoring resource allocation and costs through remote access to electronic medical records. J Oncol Pract 9(1):e13–e16. doi:10.1200/JOP.2012.000666
Bakobaki J, Joffe N, Burdett S, Tierney J, Meredith S, Stenning S (2012) A systematic search for reports of site monitoring technique comparisons in clinical trials. Clin Trials 9(6):777–780. doi:10.1177/1740774512458993
De S (2011) Hybrid approaches to clinical trial monitoring: practical alternatives to 100% source data verification. Perspect Clin Res 2(3):100–104. doi:10.4103/2229-3485.83226
Korieth K (2011) The high cost and questionable impact of 100% SDV. Cent Watch Mon 18(02)
Hullsiek KH, Kagan JM, Engen N, Grarup J, Hudson F, Denning ET, Carey C, Courtney-Rodgers D, Finley EB, Jansson PO, Pearson MT, Peavy DE, Belloso WH (2014) Investigating the efficacy of clinical trial monitoring strategies: design and implementation of the cluster randomized START monitoring substudy. Ther Innov Regul Sci. doi:10.1177/2168479014555912
Sax A, Keegan M, White D, Turner JR (2012) Risk-based monitoring: the new regulatory landscape, and conjectures on the future of clinical trial execution. J Clin Stud 4(5):26–33
Grahnen A, Karlsson K, Bragazzi F (2007) Impact of transcription errors on the outcome of a clinical trial. Clin Pharmacol Ther 81:1–29
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no competing interests.
Rights and permissions
About this article
Cite this article
Olsen, R., Bihlet, A.R., Kalakou, F. et al. The impact of clinical trial monitoring approaches on data integrity and cost—a review of current literature. Eur J Clin Pharmacol 72, 399–412 (2016). https://doi.org/10.1007/s00228-015-2004-y
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00228-015-2004-y