Abstract
Purpose
The long-term efficacy of tolvaptan, a vasopressin V2 receptor antagonist, has been reported. However, the safety of long-term treatment remains to be fully elucidated. We assessed the safety profile of tolvaptan with respect to hypernatremia.
Methods
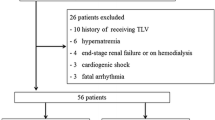
This retrospective study included 371 patients treated with tolvaptan. Risk factors for hypernatremia (serum sodium concentration ≥147 mEq/L) were determined.
Results
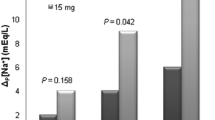
Hypernatremia occurred in 95 patients (25.6 %), of whom 71 (19.1 %) developed hypernatremia within 7 days of tolvaptan treatment (early onset). Stepwise logistic regression analysis demonstrated that baseline serum sodium ≥140 mEq/L, an initial tolvaptan dosage >7.5 mg, and a BUN/serum creatinine ratio ≥20 were independent risk factors for early onset of hypernatremia. Tolvaptan was prescribed for more than 7 days to 233 patients, of whom 123 were administrated tolvaptan for more than 1 month. Hypernatremia occurred in 24 of these patients (10.3 %) (late onset). Predictive factors for late onset of hypernatremia were an average daily dosage of tolvaptan >7.5 mg and age ≥75 years.
Conclusions
A daily dosage of 7.5 mg or less was recommended to prevent hypernatremia in short- as well as long-term tolvaptan treatment, and mainly elderly patients were at risk for hypernatremia.
Similar content being viewed by others
References
Yamamura Y, Nakamura S, Itoh S, Hirano T, Onogawa T, Yamashita T, Yamada Y, Tsujimae K, Aoyama M, Kotosai K, Ogawa H, Yamashita H, Kondo K, Tominaga M, Tsujimoto G, Mori T (1998) OPC-41061, a highly potent human vasopressin V2-receptor antagonist: pharmacological profile and aquaretic effect by single and multiple oral dosing in rats. J Pharmacol Exp Ther 287:860–867
Decaux G, Soupart A, Vassart G (2008) Non-peptide arginine-vasopressin antagonists: the vaptans. Lancet 371:1624–1632. doi:10.1016/S0140-6736(08)60695-9
Dohi K, Ito M (2014) Novel diuretic strategies for the treatment of heart failure in Japan. Circ J 78:1816–1823. doi:10.1253/circj.CJ-14-0592
Gheorghiade M, Konstam MA, Burnett JC, Grinfeld L, Maggioni AP, Swedberg K, Udelson JE, Zannad F, Cook T, Ouyang J, Zimmer C, Orlandi C, Efficacy of Vasopressin Antagonism in Heart Failure Outcome Study With Tolvaptan (EVEREST) Investigators (2007) Short-term clinical effects of tolvaptan, an oral vasopressin antagonist, in patients hospitalized for heart failure: the EVEREST Clinical Status Trials. JAMA 297:1332–1343. doi:10.1001/jama.297.12.1332
Matsuzaki M, Hori M, Izumi T, Fukunami M, Tolvaptan Investigators (2011) Efficacy and safety of tolvaptan in heart failure patients with volume overload despite the standard treatment with conventional diuretics: a phase III, randomized, double-blind, placebo-controlled study (QUEST study). Cardiovasc Drugs Ther 25:S33–S45. doi:10.1007/s10557-011-6304-x
Watanabe K, Dohi K, Sugimoto T, Yamada T, Sato Y, Ichikawa K, Sugiura E, Kumagai N, Nakamori S, Nakajima H, Hoshino K, Machida H, Okamoto S, Onishi K, Nakamura M, Nobori T, Ito M (2012) Short-term effects of low-dose tolvaptan on hemodynamic parameters in patients with chronic heart failure. J Cardiol 60:462–469. doi:10.1016/j.jjcc.2012.09.002
Schrier RW, Gross P, Gheorghiade M, Berl T, Verbalis JG, Czerwiec FS, Orlandi C (2006) Tolvaptan, a selective oral vasopressin V 2-receptor antagonist, for hyponatremia. N Engl J Med 355:2099–2112. doi:10.1056/NEJMoa065181
Kinugawa K, Sato N, Inomata T, Shimakawa T, Iwatake N, Mizuguchi K (2014) Efficacy and safety of tolvaptan in heart failure patients with volume overload. Circ J 78:844–852. doi:10.1253/circj.CJ-14-0126
Sakaida I, Yanase M, Kobayashi Y, Yasutake T, Okada M (2012) The pharmacokinetics and pharmacodynamics of tolvaptan in patients with liver cirrhosis with insufficient response to conventional diuretics: a multicentre, double-blind, parallel-group, phase III study. J Int Med Res 40:2381–2393. doi:10.1177/030006051204000637
Okita K, Kawazoe S, Hasebe C, Kajimura K, Kaneko A, Okada M, Sakaida I (2014) Dose-finding trial of tolvaptan in liver cirrhosis patients with hepatic edema: a randomized, double-blind, placebo-controlled trial. Hepatol Res 44:83–91. doi:10.1111/hepr.12099
Sakaida I, Kawazoe S, Kajimura K, Saito T, Okuse C, Takaguchi K, Okada M, Okita K (2014) Tolvaptan for improvement of hepatic edema: a phase 3, multicenter, randomized, double-blind, placebo-controlled trial. Hepatol Res 44:73–82. doi:10.1111/hepr.12098
Torres VE, Meijer E, Bae KT, Chapman AB, Devuyst O, Gansevoort RT, Grantham JJ, Higashihara E, Perrone RD, Krasa HB, Ouyang JJ, Czerwiec FS (2011) Rationale and design of the TEMPO (tolvaptan efficacy and safety in management of autosomal dominant polycystic kidney disease and its outcomes) 3-4 study. Am J Kidney Dis 57:692–699. doi:10.1053/j.ajkd.2010.11.029
Gheorghiade M, Gattis WA, O’Connor CM, Adams KF, Elkayam U, Barbagelata A, Ghali JK, Benza RL, McGrew FA, Klapholz M, Ouyang J, Orlandi C, Acute and Chronic Therapeutic Impact of a Vasopressin Antagonist in Congestive Heart Failure (ACTIV in CHF) Investigators (2004) Effects of tolvaptan, a vasopressin antagonist, in patients hospitalized with worsening heart failure: a randomized controlled trial. JAMA 291:1963–1971. doi:10.1001/jama.291.16.1963
Konstam MA, Gheorghiade M, Burnett JC, Grinfeld L, Maggioni AP, Swedberg K, Udelson JE, Zannad F, Cook T, Ouyang J, Zimmer C, Orlandi C, Efficacy of Vasopressin Antagonism in Heart Failure Outcome Study With Tolvaptan (EVEREST) Investigators (2007) Effects of oral tolvaptan in patients hospitalized for worsening heart failure: the EVEREST Outcome Trial. JAMA 297:1319–1331. doi:10.1001/jama.297.12.1319
Udelson JE, McGrew FA, Flores E, Ibrahim H, Katz S, Koshkarian G, O’Brien T, Kronenberg MW, Zimmer C, Orlandi C, Konstam MA (2007) Multicenter, randomized, double-blind, placebo-controlled study on the effect of oral tolvaptan on left ventricular dilation and function in patients with heart failure and systolic dysfunction. J Am Coll Cardiol 49:2151–2159. doi:10.1016/j.jacc.2007.01.091
Suzuki S, Yoshihisa A, Yamaki T, et al. (2013) Acute heart failure volume control multicenter randomized (AVCMA) trial: comparison of tolvaptan and carperitide. J Clin Pharmacol 53:1277–1285. doi:10.1002/jcph.197
Imamura T, Kinugawa K, Ohtani T, Sakata Y, Higo T, Kinugawa S, Tsutsui H, Sunagawa K, Komuro I (2014) Assessment of quality of life during long-term treatment of tolvaptan in refractory heart failure: design and rationale of the AQUA-TLV study. Int Heart J 55:264–267. doi:10.1536/ihj.13-326
Xiong B, Huang Y, Tan J, Yao Y, Wang C, Qian J, Rong S, Deng S, Cao Y, Zou Y, Huang J (2015) The short-term and long-term effects of tolvaptan in patients with heart failure: a meta-analysis of randomized controlled trials. Heart Fail Rev 20:633–642. doi:10.1007/s10741-015-9503-x
Matsuo S, Imai E, Horio M, Yasuda Y, Tomita K, Nitta K, Yamagata K, Tomino Y, Yokoyama H, Hishida A (2009) Revised equations for estimated GFR from serum creatinine in Japan. Am J Kidney Dis 53:982–992. doi:10.1053/j.ajkd.2008.12.034
Liamis G, Tsimihodimos V, Doumas M, Spyrou A, Bairaktari E, Elisaf M (2008) Clinical and laboratory characteristics of hypernatraemia in an internal medicine clinic. Nephrol Dial Transplant 23:136–143. doi:10.1093/ndt/gfm376
Overgaard-Steensen C, Ring T (2013) Clinical review: practical approach to hyponatraemia and hypernatraemia in critically ill patients. Crit Care 17:206. doi:10.1186/cc11805
Kim SR, Hasunuma T, Sato O, Okada T, Kondo M, Azuma J (2011) Pharmacokinetics, pharmacodynamics and safety of tolvaptan, a novel, oral, selective nonpeptide AVP V2-receptor antagonist: results of single- and multiple-dose studies in healthy Japanese male volunteers. Cardiovasc Drugs Ther 25:S5–17. doi:10.1007/s10557-011-6299-3
Inomata T, Izumi T, Matsuzaki M, Hori M, Hirayama A, Tolvaptan Investigators (2011) Phase III clinical pharmacology study of tolvaptan. Cardiovasc Drugs Ther 25:S57–S65. doi:10.1007/s10557-011-6349-x
Shoaf SE, Bricmont P, Mallikaarjun S (2013) Pharmacokinetics and pharmacodynamics of oral tolvaptan in patients with varying degrees of renal function. Kidney Int 85:953–961. doi:10.1038/ki.2013.350
Kida K, Shibagaki Y, Tominaga N, Matsumoto N, Akashi YJ, Miyake F, Kimura K (2015) Efficacy of tolvaptan added to furosemide in heart failure patients with advanced kidney dysfunction: a pharmacokinetic and pharmacodynamic study. Clin Pharmacokinet 54:273–284. doi:10.1007/s40262-014-0194-6
Kinugawa K, Inomata T, Sato N, Yasuda M, Shimakawa T, Bando K, Mizuguchi K (2015) Effectiveness and adverse events of tolvaptan in octogenarians with heart failure. Int Heart J 56:137–143. doi:10.1536/ihj.14-332
Authors’ contributions
Hirai K, Moriwaki H, Tsuji D, Inoue K, Kadoiri T and Itoh K designed and managed this study. Hirai K, Shimomura T, Moriwaki H, Ishii H, and Shimoshikiryo T collected the clinical data. Hirai K analyzed all data and wrote the manuscript. All authors read and approved the final manuscript.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
All authors declare that they had no support from any organization for the submitted work, no financial relationship with any organizations that might have an interest in the submitted work, and no other relationships or activities that could appear to have influenced the submitted work.
Ethical approval
This study was approved by the ethics committee of the Shizuoka General Hospital (Shizuoka, Japan).
Rights and permissions
About this article
Cite this article
Hirai, K., Shimomura, T., Moriwaki, H. et al. Risk factors for hypernatremia in patients with short- and long-term tolvaptan treatment. Eur J Clin Pharmacol 72, 1177–1183 (2016). https://doi.org/10.1007/s00228-016-2091-4
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00228-016-2091-4