Abstract
Purpose
The aim of this study is to differentiate recurrent/residual gliomas from postradiation changes using arterial spin labeling (ASL) perfusion and diffusion tensor imaging (DTI)-derived metrics.
Methods
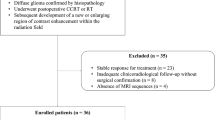
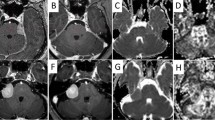
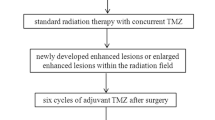
Prospective study was conducted upon 42 patients with high-grade gliomas after radiotherapy only or prior to other therapies that underwent routine MR imaging, ASL, and DTI. The tumor blood flow (TBF), fractional anisotropy (FA), and mean diffusivity (MD) of the enhanced lesion and related edema were calculated. The lesion was categorized as recurrence/residual or postradiation changes.
Results
There was significant differences between residual/recurrent gliomas and postradiation changes of TBF (P = 0.001), FA (P = 0.001 and 0.04), and MD (P = 0.001) of enhanced lesion and related edema respectively. The area under the curve (AUC) of TBF of enhanced lesion and related edema used to differentiate residual/recurrent gliomas from postradiation changes were 0.95 and 0.93 and of MD were 0.95 and 0.81 and of FA were 0.81 and 0.695, respectively. Combined ASL and DTI metrics of the enhanced lesion revealed AUC of 0.98, accuracy of 95%, sensitivity of 93.8%, specificity of 95.8%, positive predictive value (PPV) of 93.8%, and negative predictive value (NPV) of 95.8%. Combined metrics of ASL and DTI of related edema revealed AUC of 0.97, accuracy of 92.5%, sensitivity of 93.8%, specificity of 91.7%, PPV of 88.2%, and NPV of 95.7.
Conclusion
Combined ASL and DTI metrics of enhanced lesion and related edema are valuable noninvasive tools in differentiating residual/recurrent gliomas from postradiation changes.






Similar content being viewed by others
Abbreviations
- ASL:
-
Arterial spin labeling
- AUC:
-
Area under the curve
- DTI:
-
Diffusion tensor imaging
- FA:
-
Fractional anisotropy
- MD:
-
Mean diffusivity
- NPV:
-
Negative predictive value
- PPV:
-
Positive predictive value
- TBF:
-
Tumor blood flow
References
Galldiks N, Kocher M, Langen KJ (2017) Pseudoprogression after glioma therapy: an update. Expert Rev Neurother 7:1–7
Huang R (2017) Response assessment in high-grade glioma: tumor volume as endpoint. Neuro Oncol 19:744–745
Hyare H, Thust S, Rees J (2017) Advanced MRI techniques in the monitoring of treatment of gliomas. Curr Treat Options Neurol 19(3):11. https://doi.org/10.1007/s11940-017-0445-6
Shiroishi MS, Boxerman JL, Pope WB (2016) Physiologic MRI for assessment of response to therapy and prognosis in glioblastoma. Neuro Oncol 18:467–478
Dalesandro MF, Andre JB (2016) Posttreatment evaluation of brain gliomas. Neuroimaging Clin N Am 26(4):581–599. https://doi.org/10.1016/j.nic.2016.06.007
Telles BA, D Amore F, Lerner A, Law M, Shiroishi MS (2015) Imaging of the posttherapeutic brain. Top Magn Reson Imaging 24(3):147–154. https://doi.org/10.1097/RMR.0000000000000051
Abdulla S, Saada J, Johnson G, Jefferies S, Ajithkumar T (2015) Tumour progression or pseudoprogression? A review of post-treatment radiological appearances of glioblastoma. Clin Radiol 70:1299–1312
Nguyen HS, Milbach N, Hurrell SL, Cochran E, Connelly J, Bovi JA, Schultz CJ, Mueller WM, Rand SD, Schmainda KM, LaViolette PS (2016) Progressing bevacizumab-induced diffusion restriction is associated with coagulative necrosis surrounded by viable tumor and decreased overall survival in patients with recurrent glioblastoma. AJNR Am J Neuroradiol 37(12):2201–2208. https://doi.org/10.3174/ajnr.A4898
Artzi M, Bokstein F, Blumenthal DT, Aizenstein O, Liberman G, Corn BW, Ben Bashat D (2014) Differentiation between vasogenic-edema versus tumor-infiltrative area in patients with glioblastoma during bevacizumab therapy: a longitudinal MRI study. Eur J Radiol 83(7):1250–1256. https://doi.org/10.1016/j.ejrad.2014.03.026
Kerkhof M, Hagenbeek RE, van der Kallen BF, Lycklama À, Nijeholt GJ, Dirven L, Taphoorn MJ et al (2016) Interobserver variability in the radiological assessment of magnetic resonance imaging (MRI) including perfusion MRI in glioblastoma multiforme. Eur J Neurol 23(10):1528–1533. https://doi.org/10.1111/ene.13070
Fatterpekar GM, Galheigo D, Narayana A, Johnson G, Knopp E (2012) Treatment-related change versus tumor recurrence in high-grade gliomas: a diagnostic conundrum—use of dynamic susceptibility contrast-enhanced (DSC) perfusion MRI. AJR Am J Roentgenol 198(1):19–26. https://doi.org/10.2214/AJR.11.7417
Bisdas S, Naegele T, Ritz R, Pfannenberg C, Reimold M, Koh TS et al (2011) Distinguishing recurrent high-grade gliomas from radiation injury: a pilot study using dynamic contrast-enhanced MR imaging. Acad Radiol 18(5):575–583. https://doi.org/10.1016/j.acra.2011.01.018
Chuang MT, Liu YS, Tsai YS, Chen YC, Wang CK (2016) Differentiating radiation-induced necrosis from recurrent brain tumor using MR perfusion and spectroscopy: a meta-analysis. PLoS One 11(1):e0141438. https://doi.org/10.1371/journal.pone.0141438
Yoon RG, Kim HS, Koh MJ, Shim WH, Jung SC, Kim SJ, Kim JH (2017) Differentiation of recurrent glioblastoma from delayed radiation necrosis by using voxel-based multiparametric analysis of MR imaging data. Radiology 285(1):206–213. https://doi.org/10.1148/radiol.2017161588
Yoon RG, Kim HS, Paik W, Shim WH, Kim SJ, Kim JH (2017) Different diagnostic values of imaging parameters to predict pseudoprogression in glioblastoma subgroups stratified by MGMT promoter methylation. Eur Radiol 27(1):255–266. https://doi.org/10.1007/s00330-016-4346-y
Bulik M, Kazda T, Slampa P, Jancalek R (2015) The diagnostic ability of follow-up imaging biomarkers after treatment of glioblastoma in the temozolomide era: implications from proton MR spectroscopy and apparent diffusion coefficient mapping. Biomed Res Int 2015:641023. https://doi.org/10.1155/2015/641023
Prah MA, Al-Gizawiy MM, Mueller WM, Cochran EJ, Hoffmann RG, Connelly JM, Schmainda KM (2017) Spatial discrimination of glioblastoma and treatment effect with histologically-validated perfusion and diffusion magnetic resonance imaging metrics. J Neuro-Oncol. https://doi.org/10.1007/s11060-017-2617-3
Hojjati M, Badve C, Garg V, Tatsuoka C, Rogers L, Sloan A, Faulhaber P, Ros PR, Wolansky LJ (2017) Role of FDG-PET/MRI, FDG-PET/CT, and dynamic susceptibility contrast perfusion MRI in differentiating radiation necrosis from tumor recurrence in glioblastomas. J Neuroimaging. https://doi.org/10.1111/jon.12460
Kim HS, Goh MJ, Kim N, Choi CG, Kim SJ, Kim JH (2014) Which combination of MR imaging modalities is best for predicting recurrent glioblastoma? Study of diagnostic accuracy and reproducibility. Radiology 273(3):831–843. https://doi.org/10.1148/radiol.14132868
Fink JR, Carr RB, Matsusue E, Iyer RS, Rockhill JK, Haynor DR, Maravilla KR (2012) Comparison of 3 Tesla proton MR spectroscopy, MR perfusion and MR diffusion for distinguishing glioma recurrence from posttreatment effects. J Magn Reson Imaging 35(1):56–63. https://doi.org/10.1002/jmri.22801
Ozsunar Y, Mullins ME, Kwong K, Hochberg FH, Ament C, Schaefer PW, Gonzalez RG, Lev MH (2010) Glioma recurrence versus radiation necrosis?: a pilot comparison of arterial spin-labeled, dynamic susceptibility contrast enhanced MRI, and FDG-PET imaging. Acad Radiol 17(3):282–290. https://doi.org/10.1016/j.acra.2009.10.024
Wang P, Li J, Diao Q, Lin Y, Zhang J, Li L et al (2016) Assessment of glioma response to radiotherapy using 3D pulsed-continuous arterial spin labeling and 3D segmented volume. Eur J Radiol 85(11):1987–1992. https://doi.org/10.1016/j.ejrad.2016.08.009
Ye J, Bhagat SK, Li H, Luo X, Wang B, Liu L et al (2016) Differentiation between recurrent gliomas and radiation necrosis using arterial spin labeling perfusion imaging. Exp Ther Med 11:2432–2436
Nyberg E, Honce J, Kleinschmidt-DeMasters BK, Shukri B, Kreidler S, Nagae L (2016) Arterial spin labeling: pathologically proven superiority over conventional MRI for detection of high-grade glioma progression after treatment. Neuroradiol J 29(5):377–383. https://doi.org/10.1177/1971400916665375
Choi YJ, Kim HS, Jahng G-H, Kim SJ, Suh DC (2013) Pseudoprogression in patients with glioblastoma: added value of arterial spin labeling to dynamic susceptibility contrast perfusion MR imaging. Acta Radiol 54(4):448–454. https://doi.org/10.1177/0284185112474916
El-Serougy L, Abdel Razek AA, Ezzat A, Eldawoody H, El-Morsy A (2016) Assessment of diffusion tensor imaging metrics in differentiating low-grade from high-grade gliomas. Neuroradiol J 29(5):400–407. https://doi.org/10.1177/1971400916665382
Wang S, Martinez-Lage M, Sakai Y, Chawla S, Kim SG, Alonso-Basanta M, Lustig RA, Brem S, Mohan S, Wolf RL, Desai A, Poptani H (2016) Differentiating tumor progression from pseudoprogression in patients with glioblastomas using diffusion tensor imaging and dynamic susceptibility contrast MRI. AJNR Am J Neuroradiol 37(1):28–36. https://doi.org/10.3174/ajnr.A4474
Masch WR, Wang PI, Chenevert TL, Junck L, Tsien C, Heth JA, Sundgren PC (2016) Comparison of diffusion tensor imaging and magnetic resonance perfusion imaging in differentiating recurrent brain neoplasm from radiation necrosis. Acad Radiol 23(5):569–576. https://doi.org/10.1016/j.acra.2015.11.015
Castellano A, Donativi M, Rudà R, Junck L, Tsien C, Heth JA et al (2016) Evaluation of low-grade glioma structural changes after chemotherapy using DTI-based histogram analysis and functional diffusion maps. Eur Radiol 26(5):1263–1273. https://doi.org/10.1007/s00330-015-3934-6
Hope TR, Vardal J, Bjørnerud A, Larsson C, Arnesen MR, Salo RA, Groote IR (2015) Serial diffusion tensor imaging for early detection of radiation-induced injuries to normal-appearing white matter in high-grade glioma patients. J Magn Reson Imaging 41(2):414–423. https://doi.org/10.1002/jmri.24533
J-L X, Li Y-L, Lian J-M, Dou SW, Yan FS, Wu H et al (2010) Distinction between postoperative recurrent glioma and radiation injury using MR diffusion tensor imaging. Neuroradiology 52:1193–1199
Tensaouti F, Khalifa J, Lusque A, Plas B, Lotterie JA, Berry I, Laprie A, Cohen-Jonathan Moyal E, Lubrano V (2017) Response assessment in neuro-oncology criteria, contrast enhancement and perfusion MRI for assessing progression in glioblastoma. Neuroradiology 59(10):1013–1020. https://doi.org/10.1007/s00234-017-1899-7
Razek AA, Shabana AA, El Saied TO, Alrefey N (2017) Diffusion tensor imaging of mild-moderate carpal tunnel syndrome: correlation with nerve conduction study and clinical tests. Clin Rheumatol 36(10):2319–2324. https://doi.org/10.1007/s10067-016-3463-y
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Funding
No funding was received for this study.
Conflict of interest
The authors declare that they have no conflict of interest.
Ethical approval
All procedures performed in the studies involving human participants were in accordance with the ethical standards of the institutional and/or national research committee and with the 1964 Helsinki Declaration and its later amendments or comparable ethical standards.
Informed consent
Informed consent was obtained from all individual participants included in the study.
Electronic supplementary material
ESM 1
(DOC 48 kb)
Rights and permissions
About this article
Cite this article
Razek, A.A.K.A., El-Serougy, L., Abdelsalam, M. et al. Differentiation of residual/recurrent gliomas from postradiation necrosis with arterial spin labeling and diffusion tensor magnetic resonance imaging-derived metrics. Neuroradiology 60, 169–177 (2018). https://doi.org/10.1007/s00234-017-1955-3
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00234-017-1955-3