Abstract
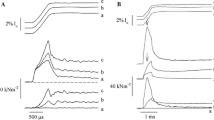
Titin (connectin) based passive force regulation has been an important physiological mechanism to adjust to varying muscle stretch conditions. Upon stretch, titin behaves as a spring capable of modulating its elastic response in accordance with changes in muscle biochemistry. One such mechanism has been the calcium-dependent stiffening of titin domains that renders the spring inherently more resistant to stretch. This transient titin-calcium interaction may serve a protective function in muscle, which could preclude costly unfolding of select domains when muscles elongate to great lengths. To test this idea, fluorescence spectroscopy was performed revealing a change in the microenvironment of the investigated immunoglobulin domain 27 (I27) of titin with calcium. Additionally, an atomic force microscope was used to evaluate the calcium-dependent regulation of passive force by stretching eight linked titin I27 domains until they unfolded. When stretching in the presence of calcium, the I27 homopolymer chain became stabilized, displaying three novel properties: (1) higher stretching forces were needed to unfold the domains, (2) the stiffness, measured as a persistence length (PL), increased and (3) the peak-to-peak distance between adjacent I27 domains increased. Furthermore, a peak order dependence became apparent for both force and PL, reflecting the importance of characterizing the dynamic unfolding history of a polymer with this approach. Together, this novel titin Ig-calcium interaction may serve to stabilize the I27 domain permitting titin to tune passive force within stretched muscle in a calcium-dependent manner.



Similar content being viewed by others
Abbreviations
- WLC:
-
Worm-like chain
- I27:
-
Titin immunoglobulin domain 27
- AFM:
-
Atomic force microscope
- PL:
-
Persistence length
References
Ainavarapu SRK, Li L, Badilla CL, Fernandez JM (2005) Ligand binding modulates the mechanical stability of dihydrofolate reductase. Biophys J 89:3337–3344. doi:10.1529/biophysj.105.062034
Albani JR (2007) Principles and applications of fluorescence spectroscopy. Oxford, UK
Anderson BR, Bogomolovas J, Labeit S, Granzier H (2010) The effects of PKCα phosphorylation on the extensibility of titin’s PEVK element. J Struct Biol 170:270–277. doi:10.1016/j.jsb.2010.02.002
Bang ML, Centner T, Fornoff F, Geach AJ, Gotthardt M, McNabb M, Witt CC, Labeit D, Gregorio CC, Granzier H, Labeit S (2001) The complete gene sequence of titin, expression of an unusual approximately 700-kDa titin isoform, and its interaction with obscurin identify a novel Z-line to I-band linking system. Circ Res 89:1065–1072. doi:10.1161/hh2301.100981
Best RB, Brockwell DJ, Toca-Herrera JL, Blake AW, Smith DA, Radford SE, Clarke J (2003a) Force mode atomic force microscopy as a tool for protein folding studies. Anal Chim Acta 479:87–105. doi:10.1016/S0003-2670(02)01572-6
Best RB, Fowler SB, Toca-Herrera JL, Steward A, Paci E, Clarke J (2003b) Mechanical unfolding of a titin Ig domain: structure of transition state revealed by combining atomic force microscopy, protein engineering and molecular dynamics simulations. J Mol Biol 330:867–877. doi:10.1016/S0022-2836(03)00618-1
Borgia A, Williams PM, Clarke J (2008) Single-molecule studies of protein folding. Annu Rev Biochem 77:101–125. doi:10.1146/annurev.biochem.77.060706.093102
Brockwell DJ, Beddard GS, Clarkson J, Zinober RC, Blake AW, Trinick J, Olmsted PD, Smith DA, Radford SE (2002) The effect of core destabilization on the mechanical resistance of I27. Biophys J 83:458–472. doi:10.1016/S0006-3495(02)75182-5
Bustamante C, Marko JF, Siggia ED, Smith S (1994) Entropic elasticity of lambda-phage DNA. Science 265:1599–1600. doi:10.1126/science.8079175
Carrion-Vazquez M, Oberhauser AF, Fowler SB, Marszalek PE, Broedel SE, Clarke J, Fernandez JM (1999) Mechanical and chemical unfolding of a single protein: a comparison. Proc Natl Acad Sci USA 96:3694–3699. doi:10.1073/pnas.96.7.3694
Coulis G, Sentandreu MA, Bleimling N, Gautel M, Benyamin Y, Ouali A (2004) Myofibrillar tightly bound calcium in skeletal muscle fibers: a possible role of this cation in titin strands aggregation. FEBS Lett 556:271–275. doi:10.1016/S0014-5793(03)01436-4
Coulis G, Becila S, Herrera-Mendez CH, Sentandreu MA, Raynaud F, Richard I, Benyamin Y, Ouali A (2008) Calpain 1 binding capacities of the N1-line region of titin are significantly enhanced by physiological concentrations of calcium. Biochem 47:9174–9183. doi:10.1021/bi800315v
Fisher TE, Oberhauser AF, Carrion-Vazquez M, Marszalek PE, Fernandez JM (1999) The study of protein mechanics with the atomic force microscope. Trends Biochem Sci 24:379–384. doi:10.1016/S0968-0004(99)01453-X
Fujita H, Labeit D, Gerull B, Labeit S, Granzier HL (2004) Titin isoform-dependent effect of calcium on passive myocardial tension. Am J Physiol Heart Circ Physiol 287:2528–2534. doi:10.1152/ajpheart.00553.2004
Gao M, Lu H, Schulten K (2001) Simulated refolding of stretched titin immunoglobulin domains. Biophys J 81:2268–2277. doi:10.1016/S0006-3495(01)75874-2
Gasteiger E, Hoogland C, Gattiker A, Duvaud S, Wilkins MR, Appel RD, Bairoch A (2005) Protein identification and analysis tools on the ExPASy server. In: Walker JM (ed) The proteomics protocols handbook. Humana Press, New York, p 571
Granzier HL, Labeit S (2006) The giant muscle protein titin is an adjustable molecular spring. Exer Sport Sci Rev 34:50–53
Granzier H, Labeit D, Wu Y, Labeit S (2002) Titin as a modular spring: emerging mechanisms for elasticity control by titin in cardiac physiology and pathophysiology. J Mus Res Cell Motil 23:457–470. doi:10.1023/A:1023458406346
Huxley AF (1957) Muscle structure and theories of contraction. Prog Biophys Biophys Chem 7:255–318
Huxley HE (1969) The mechanism of muscular contraction. Science 164:1356–1366
Huxley HE, Hanson J (1954) Changes in cross-striations of muscle during contraction and stretch and their structural implications. Nature 173:973–976
Huxley AF, Niedergerke R (1954) Structural changes in muscle during contraction. Interference microscopy of living muscle fibres. Nature 173:971–973
Improta S, Politou AS, Pastore A (1996) Immunoglobulin-like modules from titin I-band: extensible components of muscle elasticity. Structure 4:323–337. doi:10.1016/S0969-2126(96)00036-6
Joumaa V, Rassier DE, Leonard TR, Herzog W (2008) The origin of passive force enhancement in skeletal muscle. Am J Physiol Cell Physiol 294:74–78. doi:10.1152/ajpcell.00218.2007
Labeit S, Kolmerer B (1995) Titins: giant proteins in charge of muscle ultrastructure and elasticity. Science 270:293–296
Labeit D, Watanabe K, Witt C, Fujita H, Wu Y, Lahmers S, Funck T, Labeit S, Granzier H (2003) Calcium-dependent molecular spring elements in the giant protein titin. Proc Natl Acad Sci USA 100:13716–13721. doi:10.1073/pnas.2235652100
Laemmli UK (1970) Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature 227:680–685
Lakowicz JR (2006) Principles of fluorescence spectroscopy. Springer, New York
Linke WA, Fernandez J (2002) Cardiac titin: molecular basis of elasticity and cellular contribution to elastic and viscous stiffness components in myocardium. J Muscle Res Cell Motil 23:483–497. doi:10.1023/A:1023462507254
Linke WA, Grützner A (2008) Pulling single molecules of titin by AFM—recent advances and physiological implications. Pflüg Arch Eur J Physiol 456:101–115. doi:10.1007/s00424-007-0389-x
Linke WA, Rudy DE, Centner T, Gautel M, Witt C, Labeit S, Gregorio CC (1999) I-band titin in cardiac muscle is a three-element molecular spring and is critical for maintaining thin filament structure. J Cell Biol 146:631–644
Lu H, Isralewitz B, Krammer A, Vogel V, Schulten K (1998) Unfolding of titin immunoglobulin domains by steered molecular dynamics simulation. Biophys J 75:662–671. doi:10.1016/S0006-3495(98)77556-3
Marino M, Svergun DI, Kreplak L, Konarev PV, Maco B, Labeit D, Mayans O (2005) Poly-Ig tandems from I-band titin share extended domain arrangements irrespective of the distinct features of their modular constituents. J Muscle Res Cell Motil 26:355–365. doi:10.1007/s10974-005-9017-6
Marko JF, Siggia ED (1995) Stretching DNA. Macromolecules 28:8759–8770. doi:10.1021/ma00130a008
Marszalek PE, Lu H, Li H, Carrion-Vazquez M, Oberhauser AF, Schulten K, Fernandez JM (1999) Mechanical unfolding intermediates in titin modules. Nature 402:100–103. doi:10.1038/47083
Maruyama K, Murakami F, Ohashi K (1977) Connectin, an elastic protein of muscle: comparative biochemistry. J Biochem 82:339–345
Ottenheijm CAC, Granzier H (2010) Role of titin in skeletal muscle function and disease. In: Rassier DE (ed) Muscle biophysics, 1st edn. Springer, New York, pp 105–122. doi:10.1007/978-1-4419-6366-6_6
Scott KA, Steward A, Fowler SB, Clarke J (2002) Titin; a multidomain protein that behaves as the sum of its parts. J Mol Biol 315:819–829. doi:10.1006/jmbi.2001.5260
Steward A, Toca-Herrera JL, Clarke J (2002) Versatile cloning system for construction of multimeric proteins for use in atomic force microscopy. Pro Sci 11:2179–2183. doi:10.1110/ps.0212702
Tatsumi R, Maeda K, Hattori A, Takahashi K (2001) Calcium binding to an elastic portion of connectin/titin filaments. J Muscle Res Cell Motil 22:149–162. doi:10.1023/A:1010349416723
Trombitás K, Greaser M, Labeit S, Jin J, Kellermayer M, Helmes M, Granzier H (1998) Titin extensibility in situ: entropic elasticity of permanently folded and permanently unfolded molecular segments. J Cell Biol 140:853–859. doi:10.1083/jcb.140.4.853
Wang K, McCarter R, Wright J, Beverly J, Ramirez-Mitchell R (1991) Regulation of skeletal muscle stiffness and elasticity by titin isoforms: a test of the segmental extension model of resting tension. Proc Natl Acad Sci USA 88:7101–7105
Watanabe K, Muhle-Goll C, Kellermayer MSZ, Labeit S, Granzier H (2002) Different molecular mechanics displayed by titin’s constitutively and differentially expressed tandem Ig segments. J Struct Biol 137:248–258. doi:10.1006/jsbi.2002.4458
Yamasaki R, Berri M, Wu Y, Trombitás K, McNabb M, Kellermayer MSZ, Witt C, Labeit D, Labeit S, Greaser M, Granzier H (2001) Titin–actin interaction in mouse myocardium: passive tension modulation and its regulation by calcium/S100A1. Biophys J 81:2297–2313. doi:10.1016/S0006-3495(01)75876-6
Yamasaki R, Wu Y, McNabb M, Greaser M, Labeit S, Granzier H (2002) Protein kinase A phosphorylates titin’s cardiac-specific N2B domain and reduces passive tension in rat cardiac myocytes. Circ Res 90:1181–1188. doi:10.1161/01.RES.0000021115.24712.99
Zinober RC, Brockwell DJ, Beddard GS, Blake AW, Olmsted PD, Radford SE, Smith DA (2002) Mechanically unfolding proteins: the effect of unfolding history and the supramolecular scaffold. Protein Sci 11:2759–2765. doi:10.1110/ps.0224602
Acknowledgments
We thank Dr. Jane Clarke for providing us with the I27 plasmid. This work was funded by Alberta Innovates-Health Solutions, The Canadian Institutes of Health Research, Natural Sciences and Engineering Research Council of Canada, and the Canada Research Chairs Program for Molecular and Cellular Biomechanics.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
DuVall, M.M., Gifford, J.L., Amrein, M. et al. Altered mechanical properties of titin immunoglobulin domain 27 in the presence of calcium. Eur Biophys J 42, 301–307 (2013). https://doi.org/10.1007/s00249-012-0875-8
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00249-012-0875-8