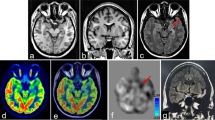
Abstract
Purpose
FDG PET is an established tool in presurgical epilepsy evaluation, but it is most often used selectively in patients with discordant MRI and EEG results. Interpretation is complicated by the presence of remote or multiple areas of hypometabolism, which leads to doubt as to the true location of the seizure onset zone (SOZ) and might have implications for predicting the surgical outcome. In the current study, we determined the sensitivity and specificity of PET localization prospectively in a consecutive unselected cohort of patients with focal epilepsy undergoing in-depth presurgical evaluation.
Methods
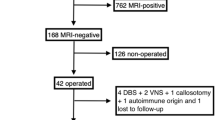
A total of 130 patients who underwent PET imaging between 2006 and 2015 matched our inclusion criteria, and of these, 86 were operated on (72% with a favourable surgical outcome, Engel class I). Areas of focal hypometabolism were identified using statistical parametric mapping and concordance with MRI, EEG and intracranial EEG was evaluated. In the surgically treated patients, postsurgical outcome was used as the gold standard for correctness of localization (minimum follow-up 12 months).
Results
PET sensitivity and specificity were both 95% in 86 patients with temporal lobe epilepsy (TLE) and 80% and 95%, respectively, in 44 patients with extratemporal epilepsy (ETLE). Significant extratemporal hypometabolism was observed in 17 TLE patients (20%). Temporal hypometabolism was observed in eight ETLE patients (18%). Among the 86 surgically treated patients, 26 (30%) had hypometabolism extending beyond the SOZ. The presence of unilobar hypometabolism, included in the resection, was predictive of complete seizure control (p = 0.007), with an odds ratio of 5.4.
Conclusion
Additional hypometabolic areas were found in one of five of this group of nonselected patients with focal epilepsy, including patients with “simple” lesional epilepsy, and this finding should prompt further in-depth evaluation of the correlation between EEG findings, semiology and PET. Hypometabolism confined to the epileptogenic zone as defined by EEG and MRI is associated with a favourable postoperative outcome in both TLE and ETLE patients.


Similar content being viewed by others
Abbreviations
- FDG:
-
18F-Fluorodeoxyglucose
- PET:
-
Positron emission tomography
- MRI:
-
Magnetic resonance imaging
- TLE:
-
Temporal lobe epilepsy
- ETLE:
-
Extratemporal lobe epilepsy
- EEG:
-
Electroencephalography
- SOZ:
-
Seizure onset zone
- SPM:
-
Statistical parametric mapping
References
Fiest KM, Sauro KM, Wiebe S, Patten SB, Kwon CS, Dykeman J, et al. Prevalence and incidence of epilepsy: a systematic review and meta-analysis of international studies. Neurology. 2017;88(3):296–303.
Pittau F, Grouiller F, Spinelli L, Seeck M, Michel CM, Vulliemoz S. The role of functional neuroimaging in pre-surgical epilepsy evaluation. Front Neurol. 2014;5:31.
Rosenow F, Luders H. Presurgical evaluation of epilepsy. Brain. 2001;124(Pt 9):1683–700.
Engel J Jr, Brown WJ, Kuhl DE, Phelps ME, Mazziotta JC, Crandall PH. Pathological findings underlying focal temporal lobe hypometabolism in partial epilepsy. Ann Neurol. 1982;12(6):518–28.
Henry TR, Roman DD. Presurgical epilepsy localization with interictal cerebral dysfunction. Epilepsy Behav. 2011;20(2):194–208.
Nelissen N, Van Paesschen W, Baete K, Van Laere K, Palmini A, Van Billoen H, et al. Correlations of interictal FDG-PET metabolism and ictal SPECT perfusion changes in human temporal lobe epilepsy with hippocampal sclerosis. Neuroimage. 2006;32(2):684–95.
Foldvary N, Lee N, Hanson MW, Coleman RE, Hulette CM, Friedman AH, et al. Correlation of hippocampal neuronal density and FDG-PET in mesial temporal lobe epilepsy. Epilepsia. 1999;40(1):26–9.
Rathore C, Dickson JC, Teotónio R, Ell P, Duncan JS. The utility of 18F-fluorodeoxyglucose PET (FDG PET) in epilepsy surgery. Epilepsy Res. 2014;108(8):1306–14.
LoPinto-Khoury C, Sperling MR, Skidmore C, Nei M, Evans J, Sharan A, et al. Surgical outcome in PET-positive, MRI-negative patients with temporal lobe epilepsy. Epilepsia. 2012;53(2):342–8.
Carne RP, O'Brien TJ, Kilpatrick CJ, MacGregor LR, Hicks RJ, Murphy MA, et al. MRI-negative PET-positive temporal lobe epilepsy: a distinct surgically remediable syndrome. Brain. 2004;127(Pt 10):2276–85.
Chassoux F, Landré E, Mellerio C, Turak B, Mann MW, Daumas-Duport C, et al. Type II focal cortical dysplasia: electroclinical phenotype and surgical outcome related to imaging. Epilepsia. 2012;53(2):349–58.
Wong CH, Bleasel A, Wen L, Eberl S, Byth K, Fulham M, et al. Relationship between preoperative hypometabolism and surgical outcome in neocortical epilepsy surgery. Epilepsia. 2012;53(8):1333–40.
Guedj E, Bonini F, Gavaret M, Trébuchon A, Aubert S, Boucekine M, et al. 18FDG-PET in different subtypes of temporal lobe epilepsy: SEEG validation and predictive value. Epilepsia. 2015;56(3):414–21.
Kumar A, Juhász C, Asano E, Sood S, Muzik O, Chugani HT. Objective detection of epileptic foci by 18F-FDG PET in children undergoing epilepsy surgery. J Nucl Med. 2010;51(12):1901–7.
Lee JJ, Kang WJ, Lee DS, Lee JS, Hwang H, Kim KJ, et al. Diagnostic performance of 18F-FDG PET and ictal 99mTc-HMPAO SPET in pediatric temporal lobe epilepsy: quantitative analysis by statistical parametric mapping, statistical probabilistic anatomical map, and subtraction ictal SPET. Seizure. 2005;14(3):213–20.
Leiderman DB, Albert P, Balish M, Bromfield E, Theodore WH. The dynamics of metabolic change following seizures as measured by positron emission tomography with fludeoxyglucose F 18. Arch Neurol. 1994;51(9):932–6.
Archambaud F, Bouilleret V, Hertz-Pannier L, Chaumet-Riffaud P, Rodrigo S, Dulac O, et al. Optimizing statistical parametric mapping analysis of 18F-FDG PET in children. EJNMMI Res. 2013;3(1):2.
Trebossen R, Comtat C, Brulon V, Bailly P, Meyer ME. Comparison of two commercial whole body PET systems based on LSO and BGO crystals respectively for brain imaging. Med Phys. 2009;36(4):1399–409.
Vargas MI, Becker M, Garibotto V, Heinzer S, Loubeyre P, Gariani J, et al. Approaches for the optimization of MR protocols in clinical hybrid PET/MRI studies. MAGMA. 2013;26(1):57–69.
Bancaud J. Surgery of epilepsy based on stereotactic investigations – the plan of the SEEG investigation. Acta Neurochir Suppl (Wien). 1980;30:25–34.
Engel J Jr, Van Ness P, Rasmussen TB, Ojemann LM. Outcome with respect to epileptic seizures. In: Engel J Jr (editor) Surgical treatment of the epilepsies. 2nd ed. New York: Raven Press; 1993. p. 609–21.
Theodore WH, Sato S, Kufta C, Balish MB, Bromfield EB, Leiderman DB. Temporal lobectomy for uncontrolled seizures: the role of positron emission tomography. Ann Neurol. 1992;32(6):789–94.
Kim SK, Lee DS, Lee SK, Kim YK, Kang KW, Chung CK, et al. Diagnostic performance of [18F]FDG-PET and ictal [99mTc]-HMPAO SPECT in occipital lobe epilepsy. Epilepsia. 2001;42(12):1531–40.
Meyer PT, Cortés-Blanco A, Pourdehnad M, Levy-Reis I, Desiderio L, Jang S, et al. Inter-modality comparisons of seizure focus lateralization in complex partial seizures. Eur J Nucl Med. 2001;28(10):1529–40.
Salanova V, Markand O, Worth R. Focal functional deficits in temporal lobe epilepsy on PET scans and the intracarotid amobarbital procedure: comparison of patients with unitemporal epilepsy with those requiring intracranial recordings. Epilepsia. 2001;42(2):198–203.
Lee SK, Lee SY, Kim KK, Hong KS, Lee DS, Chung CK. Surgical outcome and prognostic factors of cryptogenic neocortical epilepsy. Ann Neurol. 2005;58(4):525–32.
Uijl SG, Leijten FS, Arends JB, Parra J, van Huffelen AC, Moons KG. The added value of [18F]-fluoro-D-deoxyglucose positron emission tomography in screening for temporal lobe epilepsy surgery. Epilepsia. 2007;48(11):2121–9.
Kim SK, Na DG, Byun HS, Kim SE, Suh YL, Choi JY, et al. Focal cortical dysplasia: comparison of MRI and FDG-PET. J Comput Assist Tomogr. 2000;24(2):296–302.
Mendes Coelho VC, Morita ME, Amorim BJ, Ramos CD, Yasuda CL, Tedeschi H, et al. Automated online quantification method for (18)F-FDG positron emission tomography/CT improves detection of the epileptogenic zone in patients with pharmacoresistant epilepsy. Front Neurol. 2017;8:453.
Wong CH, Bleasel A, Wen L, Eberl S, Byth K, Fulham M, et al. The topography and significance of extratemporal hypometabolism in refractory mesial temporal lobe epilepsy examined by FDG-PET. Epilepsia. 2010;51(8):1365–73.
Takaya S, Hanakawa T, Hashikawa K, Ikeda A, Sawamoto N, Nagamine T, et al. Prefrontal hypofunction in patients with intractable mesial temporal lobe epilepsy. Neurology. 2006;67(9):1674–6.
Lieb JP, Dasheiff RM, Engel J Jr. Role of the frontal lobes in the propagation of mesial temporal lobe seizures. Epilepsia. 1991;32(6):822–37.
Alkonyi B, Juhász C, Muzik O, Asano E, Saporta A, Shah A, et al. Quantitative brain surface mapping of an electrophysiologic/metabolic mismatch in human neocortical epilepsy. Epilepsy Res. 2009;87(1):77–87.
Jeong JW, Asano E, Kumar Pilli V, Nakai Y, Chugani HT, Juhász C. Objective 3D surface evaluation of intracranial electrophysiologic correlates of cerebral glucose metabolic abnormalities in children with focal epilepsy. Hum Brain Mapp. 2017;38:3098–112.
Ryvlin P, Mauguière F, Sindou M, Froment JC, Cinotti L. Interictal cerebral metabolism and epilepsy in cavernous angiomas. Brain. 1995;118(Pt 3):677–87.
Spencer S, Huh L. Outcomes of epilepsy surgery in adults and children. Lancet Neurol. 2008;7(6):525–37.
Tellez-Zenteno JF, Hernández Ronquillo L, Moien-Afshari F, Wiebe S. Surgical outcomes in lesional and non-lesional epilepsy: a systematic review and meta-analysis. Epilepsy Res. 2010;89(2-3):310–8.
Radtke RA, Hanson MW, Hoffman JM, Crain BJ, Walczak TS, Lewis DV, et al. Temporal lobe hypometabolism on PET: predictor of seizure control after temporal lobectomy. Neurology. 1993;43(6):1088–92.
Knowlton RC, Laxer KD, Ende G, Hawkins RA, Wong ST, Matson GB, et al. Presurgical multimodality neuroimaging in electroencephalographic lateralized temporal lobe epilepsy. Ann Neurol. 1997;42(6):829–37.
Dupont S, Semah F, Clémenceau S, Adam C, Baulac M, Samson Y. Accurate prediction of postoperative outcome in mesial temporal lobe epilepsy: a study using positron emission tomography with 18fluorodeoxyglucose. Arch Neurol. 2000;57(9):1331–6.
Struck AF, Hall LT, Floberg JM, Perlman SB, Dulli DA. Surgical decision making in temporal lobe epilepsy: a comparison of [(18)F]FDG-PET, MRI, and EEG. Epilepsy Behav. 2011;22(2):293–7.
Higo T, Sugano H, Nakajima M, Karagiozov K, Iimura Y, Suzuki M, et al. The predictive value of FDG-PET with 3D-SSP for surgical outcomes in patients with temporal lobe epilepsy. Seizure. 2016;41:127–33.
Choi JY, Kim SJ, Hong SB, Seo DW, Hong SC, Kim BT, et al. Extratemporal hypometabolism on FDG PET in temporal lobe epilepsy as a predictor of seizure outcome after temporal lobectomy. Eur J Nucl Med Mol Imaging. 2003;30(4):581–7.
Kim DW, Lee SK, Moon HJ, Jung KY, Chu K, Chung CK. Surgical treatment of nonlesional neocortical epilepsy: long-term longitudinal study. JAMA Neurol. 2017;74(3):324–31.
Desarnaud S, Mellerio C, Semah F, Laurent A, Landre E, Devaux B, et al. (18)F-FDG PET in drug-resistant epilepsy due to focal cortical dysplasia type 2: additional value of electroclinical data and coregistration with MRI. Eur J Nucl Med Mol Imaging. 2018;45(8):1449–60.
Kim DW, Lee SK, Yun CH, Kim KK, Lee DS, Chung CK, et al. Parietal lobe epilepsy: the semiology, yield of diagnostic workup, and surgical outcome. Epilepsia. 2004;45(6):641–9.
Kogias E, Klingler JH, Urbach H, Scheiwe C, Schmeiser B, Doostkam S, et al. 3 tesla MRI-negative focal epilepsies: presurgical evaluation, postoperative outcome and predictive factors. Clin Neurol Neurosurg. 2017;163:116–20.
Sarikaya I. PET studies in epilepsy. Am J Nucl Med Mol Imaging. 2015;5(5):416–30.
Galovic M, Koepp M. Advances of molecular imaging in epilepsy. Curr Neurol Neurosci Rep. 2016;16(6):58.
Hammers A, Koepp MJ, Brooks DJ, Duncan JS. Periventricular white matter flumazenil binding and postoperative outcome in hippocampal sclerosis. Epilepsia. 2005;46(6):944–8.
Yankam Njiwa J, Bouvard S, Catenoix H, Mauguiere F, Ryvlin P, Hammers A. Periventricular [(11)C]flumazenil binding for predicting postoperative outcome in individual patients with temporal lobe epilepsy and hippocampal sclerosis. Neuroimage Clin. 2013;3:242–8.
Theodore WH, Martinez AR, Khan OI, Liew CJ, Auh S, Dustin IM, et al. PET of serotonin 1A receptors and cerebral glucose metabolism for temporal lobectomy. J Nucl Med. 2012;53(9):1375–82.
Lam J, DuBois JM, Rowley J, González-Otárula KA, Soucy JP, Massarweh G, et al. In vivo metabotropic glutamate receptor type 5 abnormalities localize the epileptogenic zone in mesial temporal lobe epilepsy. Ann Neurol. 2019;85(2):218–28.
Kagawa K, Chugani DC, Asano E, Juhász C, Muzik O, Shah A, et al. Epilepsy surgery outcome in children with tuberous sclerosis complex evaluated with alpha-[11C]methyl-L-tryptophan positron emission tomography (PET). J Child Neurol. 2005;20(5):429–38.
Bauer M, Karch R, Zeitlinger M, Liu J, Koepp MJ, Asselin MC, et al. In vivo P-glycoprotein function before and after epilepsy surgery. Neurology. 2014;83(15):1326–31.
Acknowledgments
J.T. was supported by the “Egas Moniz” grant of the Neurology Portuguese Society. M.S. was supported by SNF 113766, SNF 180365, SNF 163398. Statistical help was provided by the Clinical Research Center, Geneva University Hospitals (Prof. Thomas Perneger). The reference database was provided by the CERMEP-Imagerie du vivant, Lyon, France.
Author information
Authors and Affiliations
Contributions
J.T., M.S. and V.G. contributed to the concept and study design. M.S. and F.Picard. were responsible for patient management. J.T., F.Pittau, F.Picard, M.I.V., M.S. and V.G. contributed to data acquisition and analysis. J.T. and V.G. drafted the manuscript, tables and figures. F.Pittau, A.H., S.B., F.Picard, M.I.V., F.S. and M.S. contributed to critical revision of the manuscript. All authors approved the final version.
Corresponding author
Ethics declarations
This study was approved by the Geneva Cantonal Ethics Commission, and was in accordance with the principles of the Declaration of Helsinki and its further amendments.
Conflicts of interests
None.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
This article is part of the Topical Collection on Neurology.
Rights and permissions
About this article
Cite this article
Tomás, J., Pittau, F., Hammers, A. et al. The predictive value of hypometabolism in focal epilepsy: a prospective study in surgical candidates. Eur J Nucl Med Mol Imaging 46, 1806–1816 (2019). https://doi.org/10.1007/s00259-019-04356-x
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00259-019-04356-x