Abstract
Background
Aprepitant is a selective neurokinin-1 receptor antagonist that is effective for the prevention of nausea and vomiting caused by highly emetogenic chemotherapy. In vitro, aprepitant is a moderate inhibitor of the CYP3A4 enzyme, which is involved in the clearance of several chemotherapeutic agents. In this study we examined the potential for aprepitant to affect the pharmacokinetics and toxicity of intravenously administered docetaxel, a chemotherapeutic agent that is primarily metabolized by CYP3A4.
Methods
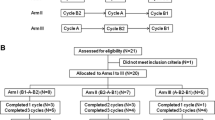
A total of 11 cancer patients (4 male, 7 female, aged 50–68 years) were enrolled in this multicenter, randomized, open-label, two-period, crossover study. Patients received a single infusion of docetaxel monotherapy, 60–100 mg/m2, on two occasions at least 3 weeks apart. During one of the cycles (treatment A), patients received docetaxel alone. During the alternate cycle (treatment B), they also received aprepitant 125 mg orally 1 h prior to docetaxel infusion (day 1), and a single oral dose of aprepitant 80 mg on days 2 and 3. The pharmacokinetic profile of docetaxel was assessed over 30 h following docetaxel infusion. Blood counts were monitored on days 1, 4, 7, and 14.
Results
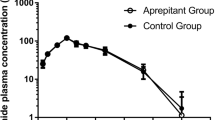
Ten patients completed the study. Concomitant administration of aprepitant did not cause any statistically or clinically significant changes in docetaxel pharmacokinetics. Values for docetaxel alone (treatment A) versus docetaxel with aprepitant (treatment B) were as follows: geometric mean AUC0–last was 3.26 vs 3.17 μg h/ml (P>0.25; ratio B/A 0.97); geometric mean AUC0–∞ 3.51 vs 3.39 μg h/ml (P>0.25; ratio B/A 0.96); geometric mean Cmax was 3.53 vs 3.37 μg/ml (P>0.25; ratio B/A 0.95); and geometric mean plasma clearance was 23.3 vs 24.2 l/h/m2 (P>0.25; ratio B/A 1.04). The corresponding harmonic mean half-life values were 10.1 and 8.5 h. The two treatment regimens had similar tolerability profiles; the median absolute neutrophil count nadirs were 681/mm3 during treatment with docetaxel alone and 975/mm3 during aprepitant coadministration.
Conclusions
Aprepitant had no clinically significant effect on either the pharmacokinetics or toxicity of standard doses of docetaxel in cancer patients. Aprepitant at clinically recommended doses may have a low potential to affect the pharmacokinetics of intravenous chemotherapeutic agents metabolized by CYP3A4.


Similar content being viewed by others
References
Poli-Bigelli S, Rodrigues-Pereira J, Carides AD, Guoguang JM, Eldridge K, Hipple A, Evans JK, Horgan KJ, Lawson F (2003) Addition of the neurokinin 1 receptor antagonist aprepitant to standard antiemetic therapy improves control of chemotherapy-induced nausea and vomiting. Results from a randomized, double-blind, placebo-controlled trial in Latin America. Cancer 97:3090–3098
Hesketh PJ, Grunberg SM, Gralla RJ, Warr DG, Roila F, de Wit R, Chawla SP, Carides AD, Ianus J, Elmer ME, Evans JK, Beck K, Reines S, Horgan KJ; Aprepitant Protocol 052 Study Group (2003) The oral neurokinin-1 antagonist aprepitant for the prevention of chemotherapy-induced nausea and vomiting: a multinational, randomized, double-blind, placebo-controlled trial in patients receiving high-dose cisplatin—the Aprepitant Protocol 052 Study Group. J Clin Oncol 21:4112–4119
de Wit R, Herrstedt J, Rapoport B, Carides AD, Guoguang-Ma J, Elmer M, Schmidt C, Evans JK, Horgan KJ (2004) The oral NK(1) antagonist, aprepitant, given with standard antiemetics provides protection against nausea and vomiting over multiple cycles of cisplatin-based chemotherapy: a combined analysis of two randomised, placebo-controlled phase III clinical trials. Eur J Cancer 40:403–410
Sanchez RI, Wang RW, Newton DJ, Bakhtiar R, Lu P, Chiu SHL, Evans DC, Huskey SEW (2004) Cytochrome P450 3A4 is the major enzyme involved in the metabolism of the substance P receptor antagonist aprepitant. Drug Metab Dispos 32:1287–1292
Majumdar AK, McCrea JB, Panebianco DL, Hesney M, Dru J, Constanzer M, Goldberg MR, Murphy G, Gottesdiener KM, Lines CR, Petty KJ, Blum RA (2003) Effects of aprepitant on cytochrome P450 3A4 activity using midazolam as a probe. Clin Pharmacol Ther 74:150–156
Bjornsson TD, Callaghan JT, Einolf HJ, Fischer V, Gan L, Grimm S, Kao J, King SP, Miwa G, Ni L, Kumar G, McLeod J, Obach RS, Roberts S, Roe A, Shah A, Snikeris F, Sullivan JT, Tweedie D, Vega JM, Walsh J, Wrighton SA (2003) The conduct of in vitro and in vivo drug-drug interaction studies: a Pharmaceutical Research and Manufacturers of America (PhRMA) perspective. Drug Metab Dispos 31:815–832
Marre F, Sanderink GJ, de Sousa G, Gaillard C, Martinet M, Rahmani R (1996) Hepatic biotransformation of docetaxel (Taxotere) in vitro: involvement of the CYP3A subfamily in humans. Cancer Res 56:1296–1302
Hirth J, Watkins PB, Strawderman M, Schott A, Bruno R, Baker LH (2000) The effect of an individual’s cytochrome CYP3A4 activity on docetaxel clearance. Clin Cancer Res 6:1255–1258
Goh BC (2002) Explaining interindividual variability of docetaxel pharmacokinetics and pharmacodynamics in Asians through phenotyping and genotyping strategies. J Clin Oncol 20:3683–3690
Yamamoto N (2004) Correlation between docetaxel clearance and estimated cytochrome P450 activity by urinary metabolite of exogenous cortisol. J Clin Oncol 18:2301–2308
Engels FK, ten Tije AJ, Baker SD, Lee CKK, Loos WJ, Vulto AG, Verweij J, Sparreboom A (2004) Effect of cytochrome P450 3A4 inhibition on the pharmacokinetics of docetaxel. Clin Pharm Ther 75:448–454
Aventis (2003) Taxotere (docetaxel) injection concentrate (package insert). Available at PDR Electronic Library: http://www.thomsonhc.com/pdrel/librarian. Accessed 5 April 2004
PPD Development (2003) Quantitation of docetaxel in human plasma via HPLC with MS/MS detection. Internal report.
Constanzer ML, Chavez-Eng CM, Dru J, Kline WF, Matuszewski BK (2004) Determination of a novel substance P inhibitor in human plasma by high-performance liquid chromatography with atmospheric pressure chemical ionization mass spectrometric detection using single and triple quadrupole detectors. J Chromatogr B 807:243–250
Hollander M, Wolfe DA (1973) Nonparametric statistical methods. John Wiley & Sons, New York
Jones B, Kenward MG (1989) The 2×2 cross-over trial with continuous data. In: Design and analysis of crossover trials. Chapman Hall, New York, pp 16–88
Zamboni WC, Egorin MJ, Van Echo DA, Day RS, Meisenberg BR, Brooks SE, Doyle LA, Nemieboka NN, Dobson JM, Tate NS, Tkaczuk KH (2000) Pharmacokinetic and pharmacodynamic study of the combination of docetaxel and topotecan in patients with solid tumors. J Clin Oncol 18:3288–3294
Shadle CR, Lee Y, Majumdar AK, Petty KJ, Gargano C, Bradstreet TE, Evans JK, Blum RA (2004) Evaluation of potential inductive effects of aprepitant on cytochrome P450 3A4 and 2C9 activity. J Clin Pharmacol 44:215–223
Slaviero KA (2004) Population pharmacokinetics of weekly docetaxel in patients with advanced cancer. Br J Clin Pharmacol 57:44–53
Clarke SJ, Rivory LP (1999) Clinical pharmacokinetics of docetaxel. Clin Pharmacokinet 36:99–114
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Nygren, P., Hande, K., Petty, K.J. et al. Lack of effect of aprepitant on the pharmacokinetics of docetaxel in cancer patients. Cancer Chemother Pharmacol 55, 609–616 (2005). https://doi.org/10.1007/s00280-004-0946-3
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00280-004-0946-3