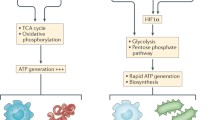
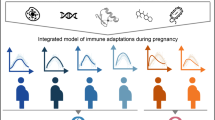
Abstract
The emerging field of immunometabolism has substantially progressed over the last years and provided pivotal insights into distinct metabolic regulators and reprogramming pathways of immune cell populations in various immunological settings. However, insights into immunometabolic reprogramming in the context of reproduction are still enigmatic. During pregnancy, the maternal immune system needs to actively adapt to the presence of the fetal antigens, i.e., by functional modifications of distinct innate immune cell subsets, the generation of regulatory T cells, and the suppression of an anti-fetal effector T cell response. Considering that metabolic pathways have been shown to affect the functional role of such immune cells in a number of settings, we here review the potential role of immunometabolism with regard to the molecular and cellular mechanisms necessary for successful reproduction. Since immunometabolism holds the potential for a therapeutic approach to alter the course of immune diseases, we further highlight how a targeted metabolic reprogramming of immune cells may be triggered by maternal anthropometric or nutritional aspects.




Similar content being viewed by others
References
Cha J, Sun X, Dey SK (2012) Mechanisms of implantation: strategies for successful pregnancy. Nat Med 18:1754–1767
Frolova AI, Moley KH (2011) Quantitative analysis of glucose transporter mRNAs in endometrial stromal cells reveals critical role of GLUT1 in uterine receptivity. Endocrinology 152:2123–2128
Kommagani R, Szwarc MM, Kovanci E et al (2013) Acceleration of the glycolytic flux by steroid receptor coactivator-2 is essential for endometrial decidualization. PLoS Genet 9:e1003900
Murdoch RN (1987) Glycolysis in the mouse uterus during the early post-implantation stages of pregnancy and the effects of acute doses of ethanol. Teratology 35:169–176
Krawczyk CM, Holowka T, Sun J et al (2010) Toll-like receptor-induced changes in glycolytic metabolism regulate dendritic cell activation. Blood 115:4742–4749
Everts B, Amiel E, Huang SC et al (2014) TLR-driven early glycolytic reprogramming via the kinases TBK1-IKKvarepsilon supports the anabolic demands of dendritic cell activation. Nat Immunol 15:323–332
Rodriguez-Prados JC, Traves PG, Cuenca J et al (2010) Substrate fate in activated macrophages: a comparison between innate, classic, and alternative activation. J Immunol 185:605–614
Donnelly RP, Loftus RM, Keating SE et al (2014) mTORC1-dependent metabolic reprogramming is a prerequisite for NK cell effector function. J Immunol 193:4477–4484
Michalek RD, Gerriets VA, Jacobs SR et al (2011) Cutting edge: distinct glycolytic and lipid oxidative metabolic programs are essential for effector and regulatory CD4+ T cell subsets. J Immunol 186:3299–3303
Gubser PM, Bantug GR, Razik L et al (2013) Rapid effector function of memory CD8+ T cells requires an immediate-early glycolytic switch. Nat Immunol 14:1064–1072
Shi LZ, Wang R, Huang G et al (2011) HIF1alpha-dependent glycolytic pathway orchestrates a metabolic checkpoint for the differentiation of TH17 and Treg cells. J Exp Med 208:1367–1376
Doughty CA, Bleiman BF, Wagner DJ et al (2006) Antigen receptor-mediated changes in glucose metabolism in B lymphocytes: role of phosphatidylinositol 3-kinase signaling in the glycolytic control of growth. Blood 107:4458–4465
Huynh A, DuPage M, Priyadharshini B et al (2015) Control of PI(3) kinase in Treg cells maintains homeostasis and lineage stability. Nat Immunol 16:188–196
Shrestha S, Yang K, Guy C et al (2015) Treg cells require the phosphatase PTEN to restrain TH1 and TFH cell responses. Nat Immunol 16:178–187
Wei J, Long L, Yang K et al (2016) Autophagy enforces functional integrity of regulatory T cells by coupling environmental cues and metabolic homeostasis. Nat Immunol 17:277–285
O’Sullivan D, van der Windt GJ, Huang SC et al (2014) Memory CD8(+) T cells use cell-intrinsic lipolysis to support the metabolic programming necessary for development. Immunity 41:75–88
Infantino V, Convertini P, Cucci L et al (2011) The mitochondrial citrate carrier: a new player in inflammation. Biochem J 438:433–436
Berod L, Friedrich C, Nandan A et al (2014) De novo fatty acid synthesis controls the fate between regulatory T and T helper 17 cells. Nat Med 20:1327–1333
Wang R, Dillon CP, Shi LZ et al (2011) The transcription factor Myc controls metabolic reprogramming upon T lymphocyte activation. Immunity 35:871–882
Gerriets VA, Kishton RJ, Nichols AG et al (2015) Metabolic programming and PDHK1 control CD4+ T cell subsets and inflammation. J Clin Invest 125:194–207
Posokhova EN, Khoshchenko OM, Chasovskikh MI et al (2008) Lipid synthesis in macrophages during inflammation in vivo: effect of agonists of peroxisome proliferator activated receptors alpha and gamma and of retinoid X receptors. Biochemistry (Mosc) 73:296–304
Wang C, Yosef N, Gaublomme J et al (2015) CD5L/AIM regulates lipid biosynthesis and restrains Th17 cell pathogenicity. Cell 163:1413–1427
Carr EL, Kelman A, GS W et al (2010) Glutamine uptake and metabolism are coordinately regulated by ERK/MAPK during T lymphocyte activation. J Immunol 185:1037–1044
Cobbold SP, Adams E, Farquhar CA et al (2009) Infectious tolerance via the consumption of essential amino acids and mTOR signaling. Proc Natl Acad Sci U S A 106:12055–12060
Yoshida R, Imanishi J, Oku T et al (1981) Induction of pulmonary indoleamine 2,3-dioxygenase by interferon. Proc Natl Acad Sci U S A 78:129–132
Zuo RJ, XW G, Qi QR et al (2015) Warburg-like glycolysis and lactate shuttle in mouse decidua during early pregnancy. J Biol Chem 290:21280–21291
Frolova AI, Moley KH (2011) Glucose transporters in the uterus: an analysis of tissue distribution and proposed physiological roles. Reproduction 142:211–220
Kim JY, Song H, Kim H et al (2009) Transcriptional profiling with a pathway-oriented analysis identifies dysregulated molecular phenotypes in the endometrium of patients with polycystic ovary syndrome. J Clin Endocrinol Metab 94:1416–1426
Frolova AI, O’Neill K, Moley KH (2011) Dehydroepiandrosterone inhibits glucose flux through the pentose phosphate pathway in human and mouse endometrial stromal cells, preventing decidualization and implantation. Mol Endocrinol 25:1444–1455
PrabhuDas M, Bonney E, Caron K et al (2015) Immune mechanisms at the maternal-fetal interface: perspectives and challenges. Nat Immunol 16:328–334
Arck PC, Hecher K (2013) Fetomaternal immune cross-talk and its consequences for maternal and offspring’s health. Nat Med 19:548–556
Apps R, Murphy SP, Fernando R et al (2009) Human leucocyte antigen (HLA) expression of primary trophoblast cells and placental cell lines, determined using single antigen beads to characterize allotype specificities of anti-HLA antibodies. Immunology 127:26–39
Madeja Z, Yadi H, Apps R et al (2011) Paternal MHC expression on mouse trophoblast affects uterine vascularization and fetal growth. Proc Natl Acad Sci U S A 108:4012–4017
Blois SM, Ilarregui JM, Tometten M et al (2007) A pivotal role for galectin-1 in fetomaternal tolerance. Nat Med 13:1450–1457
Nancy P, Tagliani E, Tay CS et al (2012) Chemokine gene silencing in decidual stromal cells limits T cell access to the maternal-fetal interface. Science 336:1317–1321
Inada K, Shima T, Ito M et al (2015) Helios-positive functional regulatory T cells are decreased in decidua of miscarriage cases with normal fetal chromosomal content. J Reprod Immunol 107:10–19
Clark DA (2016) The importance of being a regulatory T cell in pregnancy. J Reprod Immunol 116:60–69
Robertson SA, Guerin LR, Moldenhauer LM et al (2009) Activating T regulatory cells for tolerance in early pregnancy—the contribution of seminal fluid. J Reprod Immunol 83:109–116
Aluvihare VR, Kallikourdis M, Betz AG, Regulatory T (2004) Cells mediate maternal tolerance to the fetus. Nat Immunol 5:266–271
Sasaki Y, Sakai M, Miyazaki S et al (2004) Decidual and peripheral blood CD4+CD25+ regulatory T cells in early pregnancy subjects and spontaneous abortion cases. Mol Hum Reprod 10:347–353
Tilburgs T, Roelen DL, van der Mast BJ et al (2008) Evidence for a selective migration of fetus-specific CD4+CD25 bright regulatory T cells from the peripheral blood to the decidua in human pregnancy. J Immunol 180:5737–5745
Shima T, Sasaki Y, Itoh M et al (2010) Regulatory T cells are necessary for implantation and maintenance of early pregnancy but not late pregnancy in allogeneic mice. J Reprod Immunol 85:121–129
Arck P, Solano ME, Walecki M et al (2014) The immune privilege of testis and gravid uterus: same difference? Mol Cell Endocrinol 382:509–520
Rowe JH, Ertelt JM, Xin L et al (2012) Pregnancy imprints regulatory memory that sustains anergy to fetal antigen. Nature 490:102–106
Tilburgs T, Scherjon SA, van der Mast BJ et al (2009) Fetal-maternal HLA-C mismatch is associated with decidual T cell activation and induction of functional T regulatory cells. J Reprod Immunol 82:148–157
Wang WJ, Liu FJ, HM Q et al (2013) Regulation of the expression of Th17 cells and regulatory T cells by IL-27 in patients with unexplained early recurrent miscarriage. J Reprod Immunol 99:39–45
Erlebacher A, Vencato D, Price KA et al (2007) Constraints in antigen presentation severely restrict T cell recognition of the allogeneic fetus. J Clin Invest 117:1399–1411
Moldenhauer LM, Diener KR, Thring DM et al (2009) Cross-presentation of male seminal fluid antigens elicits T cell activation to initiate the female immune response to pregnancy. J Immunol 182:8080–8093
Kammerer U, Schoppet M, McLellan AD et al (2000) Human decidua contains potent immunostimulatory CD83(+) dendritic cells. Am J Pathol 157:159–169
Blois SM, Alba Soto CD, Tometten M et al (2004) Lineage, maturity, and phenotype of uterine murine dendritic cells throughout gestation indicate a protective role in maintaining pregnancy. Biol Reprod 70:1018–1023
Krey G, Frank P, Shaikly V et al (2008) In vivo dendritic cell depletion reduces breeding efficiency, affecting implantation and early placental development in mice. J Mol Med (Berl) 86:999–1011
Plaks V, Birnberg T, Berkutzki T et al (2008) Uterine DCs are crucial for decidua formation during embryo implantation in mice. J Clin Invest 118:3954–3965
Faas MM, de Vos P, Uterine NK (2017) Cells and macrophages in pregnancy. Placenta
Cartwright JE, James-Allan L, Buckley RJ et al (2017) The role of decidual NK cells in pregnancies with impaired vascular remodelling. J Reprod Immunol 119:81–84
Ratsep MT, Felker AM, Kay VR et al (2015) Uterine natural killer cells: supervisors of vasculature construction in early decidua basalis. Reproduction 149:R91–102
Evans J, Salamonsen LA, Winship A et al (2016) Fertile ground: human endometrial programming and lessons in health and disease. Nat Rev Endocrinol 12:654–667
Engler JB, Kursawe N, Solano ME et al (2017) Glucocorticoid receptor in T cells mediates protection from autoimmunity in pregnancy. Proc Natl Acad Sci U S A 114:E181–E190
Thiele K, Lydon JP, DeMayo FJ et al (2016) Specific deletion of the progesterone receptor on CD11c dendritic cells reveals a progesterone-DC-crosstalk necessary to sustain fetal development. J Reprod Immunol 115:43–43
Blois SM, Kammerer U, Alba Soto C et al (2007) Dendritic cells: key to fetal tolerance? Biol Reprod 77:590–598
Blois SM, Joachim R, Kandil J et al (2004) Depletion of CD8+ cells abolishes the pregnancy protective effect of progesterone substitution with dydrogesterone in mice by altering the Th1/Th2 cytokine profile. J Immunol 172:5893–5899
Hao S, Zhao J, Zhou J et al (2007) Modulation of 17beta-estradiol on the number and cytotoxicity of NK cells in vivo related to MCM and activating receptors. Int Immunopharmacol 7:1765–1775
Laskarin G, Tokmadzic VS, Strbo N et al (2002) Progesterone induced blocking factor (PIBF) mediates progesterone induced suppression of decidual lymphocyte cytotoxicity. Am J Reprod Immunol 48:201–209
Fournier T, Guibourdenche J, Evain-Brion D (2015) Review: hCGs: different sources of production, different glycoforms and functions. Placenta 36(Suppl 1):S60–S65
Khil LY, Jun HS, Kwon H et al (2007) Human chorionic gonadotropin is an immune modulator and can prevent autoimmune diabetes in NOD mice. Diabetologia 50:2147–2155
Schumacher A, Heinze K, Witte J et al (2013) Human chorionic gonadotropin as a central regulator of pregnancy immune tolerance. J Immunol 190:2650–2658
Schumacher A, Brachwitz N, Sohr S et al (2009) Human chorionic gonadotropin attracts regulatory T cells into the fetal-maternal Interface during early human pregnancy. J Immunol 182:5488–5497
Vacca P, Montaldo E, Croxatto D et al (2015) Identification of diverse innate lymphoid cells in human decidua. Mucosal Immunol 8:254–264
Lundell AC, Nordstrom I, Andersson K et al (2017) IFN type I and II induce BAFF secretion from human decidual stromal cells. Sci Rep 7:39904
Muzzio DO, Soldati R, Ehrhardt J et al (2014) B cell development undergoes profound modifications and adaptations during pregnancy in mice. Biol Reprod 91:115
Huang B, Faucette AN, Pawlitz MD et al (2017) Interleukin-33-induced expression of PIBF1 by decidual B cells protects against preterm labor. Nat Med 23:128–135
Mor G, Aldo P, Alvero AB (2017) The unique immunological and microbial aspects of pregnancy. Nat Rev Immunol 17:469–482
Frauwirth KA, Riley JL, Harris MH et al (2002) The CD28 signaling pathway regulates glucose metabolism. Immunity 16:769–777
MacIver NJ, Michalek RD, Rathmell JC (2013) Metabolic regulation of T lymphocytes. Annu Rev Immunol 31:259–283
Papathanassoglou E, El-Haschimi K, Li XC et al (2006) Leptin receptor expression and signaling in lymphocytes: kinetics during lymphocyte activation, role in lymphocyte survival, and response to high fat diet in mice. J Immunol 176:7745–7752
van der Windt GJ, Everts B, Chang CH et al (2012) Mitochondrial respiratory capacity is a critical regulator of CD8+ T cell memory development. Immunity 36:68–78
Rodriguez PC, Quiceno DG, Ochoa AC (2007) L-arginine availability regulates T-lymphocyte cell-cycle progression. Blood 109:1568–1573
Rath M, Muller I, Kropf P et al (2014) Metabolism via arginase or nitric oxide synthase: two competing arginine pathways in macrophages. Front Immunol 5:532
Murray PJ (2016) Amino acid auxotrophy as a system of immunological control nodes. Nat Immunol 17:132–139
Monticelli LA, Buck MD, Flamar AL et al (2016) Arginase 1 is an innate lymphoid-cell-intrinsic metabolic checkpoint controlling type 2 inflammation. Nat Immunol 17:656–665
Locksley RM (2010) Asthma and allergic inflammation. Cell 140:777–783
Munn DH, Shafizadeh E, Attwood JT et al (1999) Inhibition of T cell proliferation by macrophage tryptophan catabolism. J Exp Med 189:1363–1372
Lee GK, Park HJ, Macleod M et al (2002) Tryptophan deprivation sensitizes activated T cells to apoptosis prior to cell division. Immunology 107:452–460
Palsson-McDermott EM, Curtis AM, Goel G et al (2015) Pyruvate kinase M2 regulates Hif-1alpha activity and IL-1beta induction and is a critical determinant of the warburg effect in LPS-activated macrophages. Cell Metab 21:65–80
Pearce EL, Pearce EJ (2013) Metabolic pathways in immune cell activation and quiescence. Immunity 38:633–643
Warburg O (1956) On the origin of cancer cells. Science 123:309–314
Delgoffe GM, Kole TP, Zheng Y et al (2009) The mTOR kinase differentially regulates effector and regulatory T cell lineage commitment. Immunity 30:832–844
Brown EJ, Albers MW, Shin TB et al (1994) A mammalian protein targeted by G1-arresting rapamycin-receptor complex. Nature 369:756–758
Gabardi S, Baroletti SA (2010) Everolimus: a proliferation signal inhibitor with clinical applications in organ transplantation, oncology, and cardiology. Pharmacotherapy 30:1044–1056
Mayer CT, Berod L, Sparwasser T (2012) Layers of dendritic cell-mediated T cell tolerance, their regulation and the prevention of autoimmunity. Front Immunol 3:183
Zeng H, Yang K, Cloer C et al (2013) mTORC1 couples immune signals and metabolic programming to establish T(reg)-cell function. Nature 499:485–490
Procaccini C, Carbone F, Di Silvestre D et al (2016) The proteomic landscape of human ex vivo regulatory and conventional T cells reveals specific metabolic requirements. Immunity 44:406–421
Beier UH, Angelin A, Akimova T et al (2015) Essential role of mitochondrial energy metabolism in Foxp3(+) T-regulatory cell function and allograft survival. FASEB J 29:2315–2326
Almeida L, Lochner M, Berod L et al (2016) Metabolic pathways in T cell activation and lineage differentiation. Semin Immunol 28:514–524
Burzyn D, Benoist C, Mathis D, Regulatory T (2013) Cells in nonlymphoid tissues. Nat Immunol 14:1007–1013
Newton R, Priyadharshini B, Turka LA (2016) Immunometabolism of regulatory T cells. Nat Immunol 17:618–625
Procaccini C, De Rosa V, Galgani M et al (2010) An oscillatory switch in mTOR kinase activity sets regulatory T cell responsiveness. Immunity 33:929–941
Yin Y, Choi SC, Xu Z et al (2015) Normalization of CD4+ T cell metabolism reverses lupus. Sci Transl Med 7:274ra218
Yang Z, Shen Y, Oishi H et al (2016) Restoring oxidant signaling suppresses proarthritogenic T cell effector functions in rheumatoid arthritis. Sci Transl Med 8:331ra338
Bednarski JJ, Warner RE, Rao T et al (2003) Attenuation of autoimmune disease in Fas-deficient mice by treatment with a cytotoxic benzodiazepine. Arthritis Rheum 48:757–766
Sun Y, Tian T, Gao J et al (2016) Metformin ameliorates the development of experimental autoimmune encephalomyelitis by regulating T helper 17 and regulatory T cells in mice. J Neuroimmunol 292:58–67
Gatza E, Wahl DR, Opipari AW et al (2011) Manipulating the bioenergetics of alloreactive T cells causes their selective apoptosis and arrests graft-versus-host disease. Sci Transl Med 3:67ra68
Lee CF, Lo YC, Cheng CH et al (2015) Preventing allograft rejection by targeting immune metabolism. Cell Rep 13:760–770
Nguyen HD, Chatterjee S, Haarberg KM et al (2016) Metabolic reprogramming of alloantigen-activated T cells after hematopoietic cell transplantation. J Clin Invest 126:1337–1352
Glick GD, Rossignol R, Lyssiotis CA et al (2014) Anaplerotic metabolism of alloreactive T cells provides a metabolic approach to treat graft-versus-host disease. J Pharmacol Exp Ther 351:298–307
Cohen S, Danzaki K, MacIver NJ (2017) Nutritional effects on T-cell immunometabolism. Eur J Immunol 47:225–235
Procaccini C, De Rosa V, Galgani M et al (2012) Leptin-induced mTOR activation defines a specific molecular and transcriptional signature controlling CD4+ effector T cell responses. J Immunol 189:2941–2953
Wilk S, Scheibenbogen C, Bauer S, Jenke A, Rother M, Guerreiro M, Kudernatsch R, Goerner N, Poller W, Elligsen-Merkel D, Utku N, Magrane J, Volk HD, Skurk C (2011) Adiponectin is a negative regulator of antigen-activated T cells. Eur J Immunol 41:2323–2332
Piccio L, Cantoni C, Henderson JG et al (2013) Lack of adiponectin leads to increased lymphocyte activation and increased disease severity in a mouse model of multiple sclerosis. Eur J Immunol 43:2089–2100
Dobner J, Kaser S (2017) Body mass index and the risk of infection—from underweight to obesity. Clin Microbiol Infect. https://doi.org/10.1016/j.cmi.2017.02.013
Ahima RS, Prabakaran D, Mantzoros C et al (1996) Role of leptin in the neuroendocrine response to fasting. Nature 382:250–252
Balsells M, Garcia-Patterson A, Corcoy R (2016) Systematic review and meta-analysis on the association of prepregnancy underweight and miscarriage. Eur J Obstet Gynecol Reprod Biol 207:73–79
Huh JY, Park YJ, Ham M et al (2014) Crosstalk between adipocytes and immune cells in adipose tissue inflammation and metabolic dysregulation in obesity. Molecules and Cells 37:365–371
Yang H, Youm YH, Vandanmagsar B et al (2010) Obesity increases the production of proinflammatory mediators from adipose tissue T cells and compromises TCR repertoire diversity: implications for systemic inflammation and insulin resistance. J Immunol 185:1836–1845
Nishimura S, Manabe I, Nagasaki M et al (2009) CD8+ effector T cells contribute to macrophage recruitment and adipose tissue inflammation in obesity. Nat Med 15:914–920
Han JM, Patterson SJ, Speck M et al (2014) Insulin inhibits IL-10-mediated regulatory T cell function: implications for obesity. J Immunol 192:623 LP–623629
Mattioli B, Straface E, Quaranta MG et al (2005) Leptin promotes differentiation and survival of human dendritic cells and licenses them for Th1 priming. J Immunol 174:6820–6828
Lauterbach MA, Wunderlich FT (2017) Macrophage function in obesity-induced inflammation and insulin resistance. Pflugers Arch 469:385–396
Rocha VZ, Folco EJ, Sukhova G et al (2008) Interferon-gamma, a Th1 cytokine, regulates fat inflammation: a role for adaptive immunity in obesity. Circ Res 103:467–476
Keane KN, Calton EK, Carlessi R et al. (2017) The bioenergetics of inflammation: insights into obesity and type 2 diabetes. Eur J Clin Nutr
Matjila MJ, Hoffman A, van der Spuy ZM (2017) Medical conditions associated with recurrent miscarriage—is BMI the tip of the iceberg? Eur J Obstet Gynecol Reprod Biol 214:91–96
Bhandari HM, Tan BK, Quenby S (2016) Superfertility is more prevalent in obese women with recurrent early pregnancy miscarriage. BJOG 123:217–222
Boonstra A, Barrat FJ, Crain C et al (2001) 1α,25-dihydroxyvitamin D3 has a direct effect on naive CD4+ T Cells to enhance the development of Th2 cells. J Immunol 167:4974 LP–4974980
Gorman S, Kuritzky LA, Judge MA et al (2007) Topically applied 1,25-dihydroxyvitamin D3 enhances the suppressive activity of CD4+ CD25+ cells in the draining lymph nodes. J Immunol 179:6273–6283
Kinoshita M, Kayama H, Kusu T et al (2012) Dietary folic acid promotes survival of Foxp3+ regulatory T cells in the colon. J Immunol 189:2869–2878
Griffin MD, Lutz WH, Phan VA et al (2000) Potent inhibition of dendritic cell differentiation and maturation by vitamin D analogs. Biochem Biophys Res Commun 270:701–708
Griffin MD, Lutz W, Phan VA et al (2001) Dendritic cell modulation by 1alpha,25 dihydroxyvitamin D3 and its analogs: a vitamin D receptor-dependent pathway that promotes a persistent state of immaturity in vitro and in vivo. Proc Natl Acad Sci U S A 98:6800–6805
Troen AM, Mitchell B, Sorensen B et al (2006) Unmetabolized folic acid in plasma is associated with reduced natural killer cell cytotoxicity among postmenopausal women. J Nutr 136:189–194
Shaat N, Ignell C, Katsarou A et al. (2017) Glucose homeostasis, beta cell function, and insulin resistance in relation to vitamin D status after gestational diabetes mellitus. Acta Obstet Gynecol Scand. n/a-n/a
Lu M, Xu Y, Lv L et al (2016) Association between vitamin D status and the risk of gestational diabetes mellitus: a meta-analysis. Arch Gynecol Obstet 293:959–966
Smith TA, Kirkpatrick DR, Kovilam O et al (2015) Immunomodulatory role of vitamin D in the pathogenesis of preeclampsia. Expert Rev Clin Immunol 11:1055–1063
Murthi P, Yong HEJ, Ngyuen TPH et al. (2016) Role of the placental vitamin D receptor in modulating feto-placental growth in fetal growth restriction and preeclampsia-affected pregnancies. In 43–43
McKay JA, Xie L, Adriaens M et al. (2016) Organ-specific gene expression changes in the fetal liver and placenta in response to maternal folate depletion. Nutrients 8.
Lehr S, Hartwig S, Sell H (2012) Adipokines: a treasure trove for the discovery of biomarkers for metabolic disorders. PROTEOMICS – Clinical Applications 6:91–101
Blüher M, Mantzoros CS (2015) From leptin to other adipokines in health and disease: facts and expectations at the beginning of the 21st century. Metabolism 64:131–145
Perez-Perez A, Vilarino-Garcia T, Fernandez-Riejos P et al. Role of leptin as a link between metabolism and the immune system. Cytokine Growth Factor Rev 2017:
Macdonald SPJ, Bosio E, Neil C et al (2017) Resistin and NGAL are associated with inflammatory response, endothelial activation and clinical outcomes in sepsis. Inflamm Res 66:611–619
White RT, Damm D, Hancock N et al (1992) Human adipsin is identical to complement factor D and is expressed at high levels in adipose tissue. J Biol Chem 267:9210–9213
Procaccini C, Galgani M, De Rosa V et al (2012) Intracellular metabolic pathways control immune tolerance. Trends Immunol 33:1–7
Mor G, Cardenas I, Abrahams V, Guller S (2011) Inflammation and pregnancy: the role of the immune system at the implantation site. Ann N Y Acad Sci 1221:80–87
Dekel N, Gnainsky Y, Granot I et al (2010) Inflammation and implantation. Am J Reprod Immunol 63:17–21
Malik NM, Carter ND, Murray JF et al (2001) Leptin requirement for conception, implantation, and gestation in the mouse. Endocrinology 142:5198–5202
Io M (2009) Weight gain during pregnancy: reexamining the guidelines. National Academies Press, Washington, DC
Gabriel G, Arck PC (2014) Sex, immunity and influenza. J Infect Dis 209(Suppl 3):S93–S99
Engels G, Hierweger AM, Hoffmann J et al (2017) Pregnancy-related immune adaptation promotes the emergence of highly virulent H1N1 influenza virus strains in allogenically pregnant mice. Cell Host Microbe 21:321–333
van Riel D, Mittrucker HW, Engels G et al (2016) Influenza pathogenicity during pregnancy in women and animal models. Semin Immunopathol 38:719–726
Bapat SP, Myoung Suh J, Fang S et al (2015) Depletion of fat-resident Treg cells prevents age-associated insulin resistance. Nature 528:137–141
Feuerer M, Herrero L, Cipolletta D et al (2009) Lean, but not obese, fat is enriched for a unique population of regulatory T cells that affect metabolic parameters. Nat Med 15:930–939
Winer DA, Winer S, Shen L et al (2011) B cells promote insulin resistance through modulation of T cells and production of pathogenic IgG antibodies. Nat Med 17:610–U134
Dahlgren J (2006) Pregnancy and insulin resistance. Metab Syndr Relat Disord 4:149–152
Silha JV, Krsek M, Skrha JV et al (2003) Plasma resistin, adiponectin and leptin levels in lean and obese subjects: correlations with insulin resistance. Eur J Endocrinol 149:331–335
Steppan CM, Bailey ST, Bhat S et al (2001) The hormone resistin links obesity to diabetes. Nature 409:307–312
Degawa-Yamauchi M, Bovenkerk JE, Juliar BE et al (2003) Serum resistin (FIZZ3) protein is increased in obese humans. J Clin Endocrinol Metab 88:5452–5455
Kuzmicki M, Telejko B, Szamatowicz J et al (2009) High resistin and interleukin-6 levels are associated with gestational diabetes mellitus. Gynecol Endocrinol 25:258–263
Siddiqui K, P George T. Resistin (2017) Role in development of gestational diabetes mellitus. Biomark Med. 11:
Lobo TF, Torloni MR, Gueuvoghlanian-Silva BY et al (2013) Resistin concentration and gestational diabetes: a systematic review of the literature. J Reprod Immunol 97:120–127
Sivakumar K, Bari MF, Adaikalakoteswari A et al (2013) Elevated fetal adipsin/acylation-stimulating protein (ASP) in obese pregnancy: novel placental secretion via Hofbauer cells. J Clin Endocrinol Metab 98:4113–4122
Lo JC, Ljubicic S, Leibiger B et al (2014) Adipsin is an adipokine that improves β cell function in diabetes. Cell 158:41–53
Henson MC, Swan KF, O'Neil JS (1998) Expression of placental leptin and leptin receptor transcripts in early pregnancy and at term. Obstet Gynecol 92:1020–1028
Hassink SG, de Lancey E, Sheslow DV et al (1997) Placental leptin: an important new growth factor in intrauterine and neonatal development? Pediatrics 100:E1
Cameo P, Bischof P, Calvo JC (2003) Effect of leptin on progesterone, human chorionic gonadotropin, and interleukin-6 secretion by human term trophoblast cells in culture. Biol Reprod 68:472–477
Rice GE (2001) Cytokines and the initiation of parturition. Front Horm Res 27:113–146
Maymo JL, Perez AP, Sanchez-Margalet V et al (2009) Up-regulation of placental leptin by human chorionic gonadotropin. Endocrinology 150:304–313
Cella F, Giordano G, Cordera R (2000) Serum leptin concentrations during the menstrual cycle in normal-weight women: effects of an oral triphasic estrogen-progestin medication. Eur J Endocrinol 142:174–178
Abelenda M, Puerta M (2004) Leptin release is decreased in white adipocytes isolated from progesterone-treated rats. Endocr Res 30:335–342
Zhao M, Chen YH, Chen X, Dong XT, Zhou J, Wang H, Wu SX, Zhang C, Xu DX (2014) Folic acid supplementation during pregnancy protects against lipopolysaccharide-induced neural tube defects in mice. Toxicol Lett 224:201–208
Antony AC (2007) In utero physiology: role of folic acid in nutrient delivery and fetal development. Am J Clin Nutr 85:598S–603S
Maggini S, Wintergerst ES, Beveridge S, Hornig DH (2007) Selected vitamins and trace elements support immune function by strengthening epithelial barriers and cellular and humoral immune responses. Br J Nutr 98(Suppl 1):S29–S35
Håberg SE, London SJ, Stigum H, Nafstad P, Nystad W (2009) Folic acid supplements in pregnancy and early childhood respiratory health. Arch Dis Child 94:180–184
Courtemanche C, Elson-Schwab I, Mashiyama ST et al (2004) Folate deficiency inhibits the proliferation of primary human CD8+ T lymphocytes in vitro. J Immunol 173:3186 LP–3183192
Yamaguchi T, Hirota K, Nagahama K et al (2007) Control of immune responses by antigen-specific regulatory T cells expressing the folate receptor. Immunity 27:145–159
Who F (2004) Vitamin and mineral requirements in human nutrition, 2nd edn. World Health Organization, Geneva
Caprio M, Infante M, Calanchini M et al (2017) Vitamin D: not just the bone. Evidence for beneficial pleiotropic extraskeletal effects. Eat Weight Disord 22:27–41
Tamblyn JA, Hewison M, Wagner CL et al (2015) Immunological role of vitamin D at the maternal-fetal interface. J Endocrinol 224:R107–R121
Nunn JD, Katz DR, Barker S et al (1986) Regulation of human tonsillar T-cell proliferation by the active metabolite of vitamin D3. Immunology 59:479–484
Vanherwegen AS, Gysemans C, Mathieu C (2017) Vitamin D endocrinology on the cross-road between immunity and metabolism. Mol Cell Endocrinol 453:52–67
Alemzadeh R, Kichler J, Babar G et al (2008) Hypovitaminosis D in obese children and adolescents: relationship with adiposity, insulin sensitivity, ethnicity, and season. Metabolism 57:183–191
Kaushal M, Magon N, Vitamin D (2013) In pregnancy: a metabolic outlook. Indian J Endocrinol Metab 17:76–82
Diemert A, Lezius S, Pagenkemper M et al (2016) Maternal nutrition, inadequate gestational weight gain and birth weight: results from a prospective birth cohort. BMC Pregnancy Childbirth 16:224
Wu G, Bazer FW, Davis TA et al (2009) Arginine metabolism and nutrition in growth, health and disease. Amino Acids 37:153–168
Intestinal Mucosal WG (1998) Amino acid catabolism. J Nutr 128:1249–1252
Mills C (2012) M1 and M2 macrophages: oracles of health and disease. Crit Rev Immunol 32:463–488
Bronte V, Zanovello P (2005) Regulation of immune responses by L-arginine metabolism. Nat Rev Immunol 5:641–654
Munder M, Schneider H, Luckner C et al (2006) Suppression of T-cell functions by human granulocyte arginase. Blood 108:1627 LP–1621634
Munder M (2009) Arginase: an emerging key player in the mammalian immune system. Br J Pharmacol 158:638–651
Kim JY, Kang JS, Kim HM et al (2009) Inhibition of phenotypic and functional maturation of dendritic cells by manassantin a. J Pharmacol Sci 109:583–592
Simioni PU, Fernandes LGR, Tamashiro WMSC (2016) Downregulation of L-arginine metabolism in dendritic cells induces tolerance to exogenous antigen. Int J Immunopathol Pharmacol 30:44–57
Greenberg SS, Lancaster JR, Xie J et al (1997) Effects of NO synthase inhibitors, arginine-deficient diet, and amiloride in pregnant rats. Am J Physiol—Regul Integr Comp Physiol 273:R1031 LP–R10R1045
Buhimschi IR, Saade G, Chwalisz K et al (1998) The nitric oxide pathway in pre-eclampsia: pathophysiological implications. Hum Reprod Update 4:25–42
Kim YJ, Park HS, Lee HY et al (2006) Reduced l-arginine level and decreased placental eNOS activity in preeclampsia. Placenta 27:438–444
Rytlewski K, Olszanecki R, Lauterbach R et al (2006) Effects of oral L-arginine on the foetal condition and neonatal outcome in preeclampsia: a preliminary report. Basic Clin Pharmacol Toxicol 99:146–152
Rytlewski K, Olszanecki R, Lauterbach R et al (2008) Effects of oral l-arginine on the pulsatility indices of umbilical artery and middle cerebral artery in preterm labor. Eur J Obstet Gynecol Reprod Biol 138:23–28
Brown LD, Green AS, Limesand SW et al (2011) Maternal amino acid supplementation for intrauterine growth restriction. Front biosci (Scholar edition) 3:428–444
Gui S, Jia J, Niu X et al (2013) Arginine supplementation for improving maternal and neonatal outcomes in hypertensive disorder of pregnancy: a systematic review. J Renin-Angiotensin-Aldosterone Syst 15:88–96
Lukaszewski MA, Delahaye F, Vieau D et al (2012) Is the adipose tissue a key target of developmental programming of adult adiposity by maternal undernutrition? Adipocyte 1:64–67
Jaquet D, Gaboriau A, Czernichow P et al (2001) Relatively low serum leptin levels in adults born with intra-uterine growth retardation. Int J Obes Relat Metab Disord 25:491–495
Palou M, Konieczna J, Torrens JM et al (2012) Impaired insulin and leptin sensitivity in the offspring of moderate caloric-restricted dams during gestation is early programmed. J Nutr Biochem 23:1627–1639
Palou M, Priego T, Sanchez J et al (2010) Sexual dimorphism in the lasting effects of moderate caloric restriction during gestation on energy homeostasis in rats is related with fetal programming of insulin and leptin resistance. Nutr Metab (Lond) 7:69
Vickers MH1, Breier BH, Cutfield WS, Hofman PL, Gluckman PD (2000) Fetal origins of hyperphagia, obesity, and hypertension and postnatal amplification by hypercaloric nutrition. Am J Physiol Endocrinol Metab 279:E83–87
Grasemann C, Herrmann R, Starschinova J et al (2017) Effects of fetal exposure to high-fat diet or maternal hyperglycemia on L-arginine and nitric oxide metabolism in lung. Nutr Diabetes e244:7
Mayor RS, Finch KE, Zehr J, Morselli E, Neinast MD, Frank AP, Hahner LD, Wang J, Rakheja D, Palmer BF, Rosenfeld CR, Savani RC, Clegg DJ (2015) Maternal high-fat diet is associated with impaired fetal lung development. Am J Physiol Lung Cell Mol Physiol 309:L360–368
Acknowledgments
The writing of this review and reference to the authors’ own work were made possible through funding by the Deutsche Forschungsgemeinschaft (KFO296 and AR232/27-1 to P.C.A. and TH2126/1-1 to K.T.) and Special Funds for Science and Technology Development of Guangdong Province (2017A020214006 to LH.D).
Author information
Authors and Affiliations
Corresponding author
Additional information
This article is a contribution to the special issue on Dietary Control of Immunometabolism - Guest Editors: Joerg Heeren and Ludger Scheja
Rights and permissions
About this article
Cite this article
Thiele, K., Diao, L. & Arck, P.C. Immunometabolism, pregnancy, and nutrition. Semin Immunopathol 40, 157–174 (2018). https://doi.org/10.1007/s00281-017-0660-y
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00281-017-0660-y