Abstract
Objectives
To prospectively assess reproducibility, safety, and efficacy of microwave ablation (MWA) in the treatment of unresectable primary and secondary pulmonary tumors.
Methods
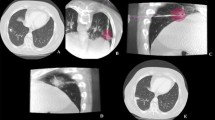
Patients with unresectable primary and metastatic lung tumors up to 4 cm were enrolled in a multicenter prospective clinical trial and underwent CT-guided MWA. Treatments were delivered using pre-defined MW power and duration settings, based on target tumor size and histology classifications. Patients were followed for up to 24 months. Treatment safety, efficacy, and reproducibility were assessed. Ablation volumes were measured at CT scan and compared with ablation volumes obtained on ex vivo bovine liver using equal treatment settings.
Results
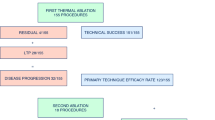
From September 2015 to September 2017, 69 MWAs were performed in 54 patients, achieving technical success in all cases and treatment completion without deviations from the standardized protocol in 61 procedures (88.4%). Immediate post-MWA CT scans showed ablation dimensions smaller by about 25% than in the ex vivo model; however, a remarkable volumetric increase (40%) of the treated area was observed at 1 month post-ablation. No treatment-related deaths nor complications were recorded. Treatments of equal power and duration yielded fairly reproducible ablation dimensions at 48-h post-MWA scans. In comparison with the ex vivo liver model, in vivo ablation sizes were systematically smaller, by about 25%. Overall LPR was 24.7%, with an average TLP of 8.1 months. OS rates at 12 and 24 months were 98.0% and 71.3%, respectively.
Conclusions
Percutaneous CT-guided MWA is a reproducible, safe, and effective treatment for malignant lung tumors up to 4 cm in size.
Key Points
• Percutaneous MWA treatment of primary and secondary lung tumors is a repeatable, safe, and effective therapeutic option.
• It provides a fairly reproducible performance on both the long and short axis of the ablation zone.
• When using pre-defined treatment duration and power settings according to tumor histology and size, LPR does not increase with increasing tumor size (up to 4 cm) for both primary and metastatic tumors.





Similar content being viewed by others
Abbreviations
- AVG:
-
Average
- CA:
-
Complete ablation
- COPD:
-
Chronic obstructive pulmonary disease
- CT:
-
Computed tomography
- CV:
-
Coefficient of variation
- D :
-
Ablation short axis (i.e., diameter perpendicular to the microwave probe)
- DP:
-
Distant progression
- DPR:
-
Distant progression rate
- E d :
-
Microwave energy delivered to the patient
- EV:
-
Ex vivo data series (i.e., ablations on ex vivo bovine liver)
- GGO:
-
Ground-glass opacity
- IV:
-
In vivo data series (i.e., ablations on MALT patients)
- L :
-
Ablation long axis (i.e., longest diameter, along the microwave probe track)
- LP:
-
Local progression
- LPFS:
-
Local progression-free survival
- LPR:
-
Local tumor progression rate
- MALT:
-
Microwave Ablation of Lung Tumors (study acronym)
- MALT1:
-
MALT study arm related to primary lung tumors
- MALT2:
-
MALT study arm related to metastatic lung tumors
- MAX:
-
Maximum value
- MIN:
-
Minimum value
- MW:
-
Microwave radiation (2450 MHz)
- MWA:
-
Microwave ablation
- NSCLC:
-
Non-small cell lung cancer
- OS:
-
Overall survival
- P :
-
Power of ablation treatment
- PA:
-
Partial ablation
- RFA:
-
Radiofrequency ablation
- S :
-
Ablation sphericity index
- SBRT:
-
Stereotactic body radiation therapy
- SD:
-
Standard deviation
- T :
-
Duration of ablation treatment
- TDP:
-
Time to distant progression
- TLP:
-
Time to local progression
- V 0 :
-
Ablation volume measured pre-MWA
- V n :
-
Normalized ablation volume
- V t :
-
Ablation volume measured at time t post-MWA
References
Prud’homme C, Deschamps F, Moulin B et al (2019) Image-guided lung metastasis ablation: a literature review. Int J Hyperthermia 36:37–45
Jiang B, Mcclure MA, Chen T, Chen S (2018) Efficacy and safety of thermal ablation of lung malignancies: a network meta-analysis. Ann Thorac Med 13:243–250
Skinner MG, Iizuka MN, Kolios MC, Sherar MD (1998) A theoretical comparison of energy sources—microwave, ultrasound and laser—for interstitial thermal therapy. Phys Med Biol 43:3535–3547
Brace CL, Hinshaw JL, Laeseke PF, Sampson LA, Lee FT (2009) Pulmonary thermal ablation: comparison of radiofrequency and microwave devices by using gross pathologic and CT findings in a swine model. Radiology 251:705–711
Aufranc V, Farouil G, Abdel-Rehim M et al (2019) Percutaneous thermal ablation of primary and secondary lung tumors: Comparison between microwave and radiofrequency ablation. Diagn Interv Imaging 100:781–791
Izzo F, Granata V, Grassi R et al (2019) Radiofrequency ablation and microwave ablation in liver tumors: an update. Oncologist. 24(10):e990–e1005. https://doi.org/10.1634/theoncologist.2018-0337
Shakeri S, Raman SS (2019) Trends in percutaneous thermal ablation therapies in the treatment of T1a renal cell carcinomas rather than partial nephrectomy/radical nephrectomy. Semin Intervent Radiol 36:183–193
Xiong L, Dupuy DE (2016) Lung ablation: what’s new? J Thorac Imaging 31:228–237
Hinshaw JL, Lubner MG, Ziemlewicz TJ, Lee FT Jr, Brace CL (2014) Percutaneous tumor ablation tools: microwave, radiofrequency, or cryoablation--what should you use and why? Radiographics 34:1344–1362
Iezzi R, Larici AR, Franchi P et al (2017) Tailoring protocols for chest CT applications: when and how? Diagn Interv Radiol 23:420–427
Anderson EM, Lees WR, Gillams AR (2009) Early indicators of treatment success after percutaneous radiofrequency of pulmonary tumors. Cardiovasc Intervent Radiol 32:478–483
de Baère T (2011) Lung tumor radiofrequency ablation: where do we stand? Cardiovasc Intervent Radiol 34:241–251
Vogl TJ, Worst TS, Naguib NN, Ackermann H, Gruber-Rouh T, Nour-Eldin NE (2013) Factors influencing local tumor control in patients with neoplastic pulmonary nodules treated with microwave ablation: a risk-factor analysis. AJR Am J Roentgenol 200(3):665–667
Khalilzadeh O, Baerlocher MO, Shyn PB et al (2017) Proposal of a new adverse event classification by the Society of Interventional Radiology Standards of Practice Committee. J Vasc Interv Radiol 28:1432–1437
Amabile C, Ahmed M, Solbiati L et al (2017) Microwave ablation of primary and secondary liver tumours: ex vivo, in vivo, and clinical characterisation. Int J Hyperthermia 33:34–42
Farina L, Nissenbaum Y, Cavagnaro M, Goldberg SN (2018) Tissue shrinkage in microwave thermal ablation: comparison of three commercial devices. Int J Hyperthermia 34:382–391
Amabile C, Farina L, Lopresto V et al (2017) Tissue shrinkage in microwave ablation of liver: an ex vivo predictive model. Int J Hyperthermia 33:101–109
Farina L, Weiss N, Nissenbaum Y et al (2014) Characterisation of tissue shrinkage during microwave thermal ablation. Int J Hyperthermia 30:419–428
Sommer CM, Sommer SA, Mokry T et al (2013) Quantification of tissue shrinkage and dehydration caused by microwave ablation: experimental study in kidneys for the estimation of effective coagulation volume. J Vasc Interv Radiol 24:1241–1248
Liu D, Brace CL (2019) Evaluation of tissue deformation during radiofrequency and microwave ablation procedures: influence of output energy delivery. Med Phys 46:4127–4134
Brace CL, Diaz TA, Hinshaw JL, Lee FT Jr (2010) Tissue contraction caused by radiofrequency and microwave ablation: a laboratory study in liver and lung. J Vasc Interv Radiol 21:1280–1286
Belfiore G, Ronza F, Belfiore MP et al (2013) Patients’ survival in lung malignancies treated by microwave ablation: our experience on 56 patients. Eur J Radiol 82:177–181
Ferguson CD, Luis CR, Steinke K (2017) Safety and efficacy of microwave ablation for medically inoperable colorectal pulmonary metastases: single-centre experience. J Med Imaging Radiat Oncol 61:243–249
Zheng A, Wang X, Yang X et al (2014) Major complications after lung microwave ablation: a single-center experience on 204 sessions. Ann Thorac Surg 98:243–248
Ierardi AM, Coppola A, Lucchina N, Carrafiello G (2017) Treatment of lung tumours with high-energy microwave ablation: a single-centre experience. Med Oncol 34:5
Wolf FJ, Grand DJ, Machan JT, DiPetrillo TA, Mayo-Smith WW, Dupuy DE (2008) Microwave ablation of lung malignancies: effectiveness, CT findings, and safety in 50 patients. Radiology 247:871–879
Healey TT, March BT, Baird G, Dupuy DE (2017) Microwave ablation for lung neoplasms: a retrospective analysis of long-term results. J Vasc Interv Radiol 28:206–211
Hiraki T, Gobara H, Iishi T et al (2007) Percutaneous radiofrequency ablation for pulmonary metastases from colorectal cancer: midterm results in 27 patients. J Vasc Interv Radiol 18:1264–1269
Yan TD, King J, Sjarif A et al (2007) Treatment failure after percutaneous radiofrequency ablation for nonsurgical candidates with pulmonary metastases from colorectal carcinoma. Ann Surg Oncol 14:1718–1726
Gillams AR, Lees WR (2008) Radiofrequency ablation of lung metastases: factors influencing success. Eur Radiol 18:672–677
Hiraki T, Sakurai J, Tsuda T et al (2006) Risk factors for local progression after percutaneous radiofrequency ablation of lung tumors: evaluation based on a preliminary review of 342 tumors. Cancer 107:2873–2880
Lu Q, Cao W, Huang L et al (2012) CT-guided percutaneous microwave ablation of pulmonary malignancies: results in 69 cases. World J Surg Oncol 10:80
Ierardi AM, Floridi C, Fontana F et al (2013) Microwave ablation of liver metastases to overcome the limitations of radiofrequency ablation. Radiol Med 118:949–961
Baisi A, De Simone M, Raveglia F, Cioffi U (2013) Thermal ablation in the treatment of lung cancer: present and future. Eur J Cardiothorac Surg 43:683–686
Funding
The authors state that this work has not received any funding.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Guarantor
The scientific guarantor of this publication is Roberto Iezzi.
Conflict of interest
One author of this manuscript (NT) declares relationships with the following companies: Nevio Tosoratti is an employee of H.S. Hospital Service SpA, the company manufacturing the MWA apparatus used in the study.
The other authors of this manuscript declare no relationships with any companies whose products or services may be related to the subject matter of the article.
Statistics and biometry
One of the authors has significant statistical expertise.
No complex statistical methods were necessary for this paper.
Informed consent
Written informed consent was obtained from all subjects (patients) in this study.
Written informed consent was waived by the Institutional Review Board.
Ethical approval
Institutional Review Board approval was obtained.
Methodology
• prospective
• case-control study/experimental study
• multicenter study
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Iezzi, R., Cioni, R., Basile, D. et al. Standardizing percutaneous Microwave Ablation in the treatment of Lung Tumors: a prospective multicenter trial (MALT study). Eur Radiol 31, 2173–2182 (2021). https://doi.org/10.1007/s00330-020-07299-2
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00330-020-07299-2