Abstract
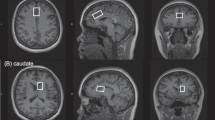
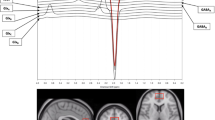
Bipolar disorder (BD) is characterized by unstable mood states ranging from mania to depression. Although there is some evidence that mood instability may result from an imbalance between excitatory glutamatergic and inhibitory GABA-ergic neurotransmission, few proton magnetic resonance spectroscopy (1H-MRS) studies have measured these two neurometabolites simultaneously in BD. The enzyme glutamic acid decarboxylase (GAD1) catalyzes the decarboxylation of glutamate (Glu) to GABA, and its single nucleotide polymorphisms (SNPs) might influence Glu/GABA ratio. Thus, we investigated Glu/GABA ratio in the dorsal anterior cingulate cortex (dACC) of euthymic BD type I patients and healthy controls (HC), and assessed the influence of both mood stabilizers and GAD1 SNPs on this ratio. Eighty-eight subjects (50 euthymic BD type I patients and 38 HC) underwent 3T 1H-MRS in the dACC (2 × 2 × 4.5 cm3) using a two-dimensional JPRESS sequence and all subjects were genotyped for 4 SNPs in the GAD1 gene. BD patients had lower dACC Glu/GABA ratio compared to HC, where this was influenced by anticonvulsant and antipsychotic medications, but not lithium. The presence of GAD1 rs1978340 allele A was associated with higher Glu/GABA ratio in BD, while patients without this allele taking mood stabilizers had a lower Glu/GABA ratio. The lowering of dACC Glu/GABA could be one explanation for the mood stabilizing action of anticonvulsants and antipsychotics in BD type I euthymia. Therefore, this putative role of Glu/GABA ratio and the influence of GAD1 genotype interacting with mood stabilization medication should be confirmed by further studies involving larger samples and other mood states.
ClincalTrials.gov registration: NCT01237158.



Similar content being viewed by others
References
Goodwin FK, Jamison KR (2007) Manic-depressive illness: bipolar and recurrent unipolar disorders, 2nd edn. Oxford University Press, New York
Stagg CJ, Bestmann S, Constantinescu AO, Moreno LM, Allman C, Mekle R et al (2011) Relationship between physiological measures of excitability and levels of glutamate and GABA in the human motor cortex. J Physiol (Lond). 589(Pt 23):5845–5855
Rossignol E (2011) Genetics and function of neocortical GABAergic interneurons in neurodevelopmental disorders. Neural Plast. 2011:649325
Yüksel C, Ongur D (2010) Magnetic resonance spectroscopy studies of glutamate-related abnormalities in mood disorders. Biol Psychiatry. 68(9):785–794
Brady RO, McCarthy JM, Prescot AP, Jensen JE, Cooper AJ, Cohen BM et al (2013) Brain gamma-aminobutyric acid (GABA) abnormalities in bipolar disorder. Bipolar Disord. 15(4):434–439
Altamura CA, Mauri MC, Ferrara A, Moro AR, D'Andrea G, Zamberlan F (1993) Plasma and platelet excitatory amino acids in psychiatric disorders. Am J Psychiatry. 150(11):1731–1733
Petty F (1994) Plasma concentrations of gamma-aminobutyric acid (GABA) and mood disorders: a blood test for manic depressive disease? Clin Chem. 40(2):296–302
Post RM, Ballenger JC, Hare TA, Goodwin FK, R LC, Jimerson DC et al (1980) Cerebrospinal fluid GABA in normals and patients with affective disorders. Brain Res Bull 5(Suppl. 2):755–759
Gerner RH, Fairbanks L, Anderson GM, Young JG, Scheinin M, Linnoila M et al (1984) CSF neurochemistry in depressed, manic, and schizophrenic patients compared with that of normal controls. Am J Psychiatry. 141(12):1533–1540
Gigante AD, Bond DJ, Lafer B, Lam RW, Young LT, Yatham LN (2012) Brain glutamate levels measured by magnetic resonance spectroscopy in patients with bipolar disorder: a meta-analysis. Bipolar Disord. 14(5):478–487
Kraguljac NV, Reid M, White D, Jones R, den Hollander J, Lowman D et al (2012) Neurometabolites in schizophrenia and bipolar disorder - a systematic review and meta-analysis. Psychiatry Res. 203(2–3):111–125
Chiapponi C, Piras F, Piras F, Caltagirone C, Spalletta G (2016) GABA system in schizophrenia and mood disorders: a mini review on third-generation imaging studies. Front Psychiatry. 7:61
Lener MS, Niciu MJ, Ballard ED, Park M, Park LT, Nugent AC et al (2017) Glutamate and gamma-aminobutyric acid systems in the pathophysiology of major depression and antidepressant response to ketamine. Biol Psychiatry. 81(10):886–897
Rubenstein JLR, Merzenich MM (2003) Model of autism: increased ratio of excitation/inhibition in key neural systems. Genes Brain Behav. 2(5):255–267
Harada M, Taki MM, Nose A, Kubo H, Mori K, Nishitani H et al (2011) Non-invasive evaluation of the GABAergic/glutamatergic system in autistic patients observed by MEGA-editing proton MR spectroscopy using a clinical 3 tesla instrument. J Autism Dev Disord. 41(4):447–454
Anticevic A, Savic A, Repovs G, Yang G, McKay DR, Sprooten E et al (2015) Ventral anterior cingulate connectivity distinguished nonpsychotic bipolar illness from psychotic bipolar disorder and schizophrenia. Schizophr Bull. 41(1):133–143
Soeiro de Souza MG, Otaduy MCG, Machado-Vieira R, Moreno RA, Nery FG, Leite C et al (2018) Anterior cingulate cortex glutamatergic metabolites and mood stabilizers in euthymic bipolar I disorder patients: a proton magnetic resonance spectroscopy study. Biol Psychiatry Cogn Neurosci Neuroimaging. https://doi.org/10.1016/j.bpsc.2018.02.007
Colla M, Schubert F, Bubner M, Heidenreich JO, Bajbouj M, Seifert F et al (2009) Glutamate as a spectroscopic marker of hippocampal structural plasticity is elevated in long-term euthymic bipolar patients on chronic lithium therapy and correlates inversely with diurnal cortisol. Mol Psychiatry 14(7):696–704, 647
Bhagwagar Z, Wylezinska M, Jezzard P, Evans J, Ashworth F, Sule A et al (2007) Reduction in occipital cortex gamma-aminobutyric acid concentrations in medication-free recovered unipolar depressed and bipolar subjects. Biol Psychiatry. 61(6):806–812
Senaratne R, Milne AM, MacQueen GM, Hall GBC (2009) Increased choline-containing compounds in the orbitofrontal cortex and hippocampus in euthymic patients with bipolar disorder: a proton magnetic resonance spectroscopy study. Psychiatry Res. 172(3):205–209
Ehrlich A, Schubert F, Pehrs C, Gallinat J (2015) Alterations of cerebral glutamate in the euthymic state of patients with bipolar disorder. Psychiatry Res. 233(2):73–80
Soeiro de Souza MG, Henning A, Machado-Vieira R, Moreno RA, Pastorello BF, da Costa Leite C et al (2015) Anterior cingulate Glutamate-Glutamine cycle metabolites are altered in euthymic bipolar I disorder. Eur Neuropsychopharmacol 25(12):2221–2229
Levy LM, Degnan AJ (2013) GABA-based evaluation of neurologic conditions: MR spectroscopy. AJNR Am J Neuroradiol. 34(2):259–265
Schür RR, Draisma LWR, Wijnen JP, Boks MP, Koevoets MGJC, Joëls M et al (2016) Brain GABA levels across psychiatric disorders: a systematic literature review and meta-analysis of (1) H-MRS studies. Hum Brain Mapp 37(9):3337–3352
Wang PW, Sailasuta N, Chandler RA, Ketter TA (2006) Magnetic resonance spectroscopy measurement of cerebral gamma-aminobutyric acid concentrations in patients with bipolar disorders. Acta Neuropsychiatr 2(18):120–126
Kaufman RE, Ostacher MJ, Marks EH, Simon NM, Sachs GS, Jensen JE et al (2009) Brain GABA levels in patients with bipolar disorder. Prog Neuropsychopharmacol Biol Psychiatry. 33(3):427–434
Godlewska BR, Yip SW, Near J, Goodwin GM, Cowen PJ (2014) Cortical glutathione levels in young people with bipolar disorder: a pilot study using magnetic resonance spectroscopy. Psychopharmacology 231(2):327–332
Prisciandaro JJ, Tolliver BK, Prescot AP, Brenner HM, Renshaw PF, Brown TR et al (2017) Unique prefrontal GABA and glutamate disturbances in co-occurring bipolar disorder and alcohol dependence. Transl Psychiatry. 7(7):e1163
Wang PW, Sailasuta N, Chandler RA, Ketter TA (2006) Magnetic resonance spectroscopic measurement of cerebral gamma-aminobutyric acid concentrations in patients with bipolar disorders. Acta Neuropsychiatr 18(2):120–126
Ford TC, Nibbs R, Crewther DP (2017) Increased glutamate/GABA+ ratio in a shared autistic and schizotypal trait phenotype termed social disorganisation. Neuroimage Clin. 16:125–131
Ford TC, Nibbs R, Crewther DP (2017) Glutamate/GABA+ ratio is associated with the psychosocial domain of autistic and schizotypal traits. PLoS ONE 12(7):e0181961
Anwyl R (1991) Modulation of vertebrate neuronal calcium channels by transmitters. Brain Res Brain Res Rev. 16(3):265–281
Erlander MG, Tillakaratne NJ, Feldblum S, Patel N, Tobin AJ (1991) Two genes encode distinct glutamate decarboxylases. Neuron 7(1):91–100
Bu DF, Erlander MG, Hitz BC, Tillakaratne NJ, Kaufman DL, Wagner-McPherson CB et al (1992) Two human glutamate decarboxylases, 65-kDa GAD and 67-kDa GAD, are each encoded by a single gene. Proc Natl Acad Sci USA 89(6):2115–2119
Guidotti A, Auta J, Davis JM, Di-Giorgi-Gerevini V, Dwivedi Y, Grayson DR et al (2000) Decrease in reelin and glutamic acid decarboxylase67 (GAD67) expression in schizophrenia and bipolar disorder: a postmortem brain study. Arch Gen Psychiatry. 57(11):1061–1069
Heckers S, Stone D, Walsh J, Shick J, Koul P, Benes FM (2002) Differential hippocampal expression of glutamic acid decarboxylase 65 and 67 messenger RNA in bipolar disorder and schizophrenia. Arch Gen Psychiatry. 59(6):521–529
Woo T-UW, Walsh JP, Benes FM (2004) Density of glutamic acid decarboxylase 67 messenger RNA-containing neurons that express the N-methyl-d-aspartate receptor subunit NR2A in the anterior cingulate cortex in schizophrenia and bipolar disorder. Arch Gen Psychiatry 61(7):649–657
Fatemi SH, Hossein Fatemi S, Stary JM, Earle JA, Araghi-Niknam M, Eagan E (2005) GABAergic dysfunction in schizophrenia and mood disorders as reflected by decreased levels of glutamic acid decarboxylase 65 and 67 kDa and Reelin proteins in cerebellum. Schizophr Res. 72(2–3):109–122
Thompson M, Weickert CS, Wyatt E, Webster MJ (2009) Decreased glutamic acid decarboxylase(67) mRNA expression in multiple brain areas of patients with schizophrenia and mood disorders. J Psychiatr Res. 43(11):970–977
Lundorf MD, Buttenschøn HN, Foldager L, Blackwood DHR, Muir WJ, Murray V et al (2005) Mutational screening and association study of glutamate decarboxylase 1 as a candidate susceptibility gene for bipolar affective disorder and schizophrenia. Am J Med Genet B Neuropsychiatr Genet. 135B(1):94–101
Chung Y-CE, Chen S-C, Chuang L-C, Shih W-L, Chiu Y-H, Lu M-L et al (2017) Evaluation of the interaction between genetic variants of GAD1 and miRNA in bipolar disorders. J Affect Disord 223:1–7
Marenco S, Savostyanova AA, van der Veen JW, Geramita M, Stern A, Barnett AS et al (2010) Genetic modulation of GABA levels in the anterior cingulate cortex by GAD1 and COMT. Neuropsychopharmacology. 35(8):1708–1717
Krystal JH, Sanacora G, Blumberg H, Anand A, Charney DS, Marek G et al (2002) Glutamate and GABA systems as targets for novel antidepressant and mood-stabilizing treatments. Mol Psychiatry. 7(Suppl 1):S71–80
Mesdjian E, Ciesielski L, Valli M, Bruguerolle B, Jadot G, Bouyard P et al (1982) Sodium valproate: kinetic profile and effects on GABA levels in various brain areas of the rat. Prog Neuropsychopharmacol Biol Psychiatry. 6(3):223–233
Pisanu C, Papadima EM, Del Zompo M, Squassina A (2018) Understanding the molecular mechanisms underlying mood stabilizer treatments in bipolar disorder: potential involvement of epigenetics. Neurosci Lett. 16(669):24–31
Gavin DP, Kartan S, Chase K, Jayaraman S, Sharma RP (2009) Histone deacetylase inhibitors and candidate gene expression: an in vivo and in vitro approach to studying chromatin remodeling in a clinical population. J Psychiatr Res. 43(9):870–876
First MB, Spitzer RL, Williams JB (1996) Structured clinical interview for DSM-IV axis I disorders SCID-I. American Psychiatric Press, Washington, DC, p 1
DSM-IV PATFO (2000) Diagnostic and statistical manual of mental disorders: DSM-IV-TR. American Psychiatric Publishing, Inc, Washington, DC
Moreira MT, Smith LA, Foxcroft D (2009) Social norms interventions to reduce alcohol misuse in university or college students. Cochrane Database Syst Rev 2009(3):CD006748. https://doi.org/10.1002/14651858.CD006748.pub2
Young RC, Biggs JT, Ziegler VE, Meyer DA (1978) A rating scale for mania: reliability, validity, and sensitivity. Br J Psychiatry. 133:429–435
Hamilton M (1967) Development of a rating scale for primary depressive illness. Br J Soc Clin Psychol. 6(4):278–296
Sheehan DV, Lecrubier Y, Sheehan KH, Amorim P, Janavs J, Weiller E et al (1998) The Mini-International Neuropsychiatric Interview (M.I.N.I.): the development and validation of a structured diagnostic psychiatric interview for DSM-IV and ICD-10. J Clin Psychiatry 59(Suppl 20):22–33 (quiz 34–57)
Schulte RF, Lange T, Beck J, Meier D, Boesiger P (2006) Improved two-dimensional J-resolved spectroscopy. NMR Biomed. 19(2):264–270
Tkác I, Starcuk Z, Choi IY, Gruetter R (1999) In vivo 1H NMR spectroscopy of rat brain at 1 ms echo time. Magn Reson Med. 41(4):649–656
Fuchs A, Boesiger P, Schulte RF, Henning A (2013) ProFit revisited. Magn Reson Med. 71(2):458–468
Schulte RF, Boesiger P (2006) ProFit: two-dimensional prior-knowledge fitting of J-resolved spectra. NMR Biomed. 19(2):255–263
Provencher SWS (1993) Estimation of metabolite concentrations from localized in vivo proton NMR spectra. Magn Reson Med. 30(6):672–679
van der Veen JW, de Beer R, Luyten PR, van Ormondt D (1988) Accurate quantification of in vivo 31P NMR signals using the variable projection method and prior knowledge. Magn Reson Med. 6(1):92–98
Smith SA, Levante TO, de Beer R, Luyten PR, van Ormondt D (1994) Computer simulations in magnetic resonance. An object-oriented programming approach. J Magn Reson A 106:75–105. https://doi.org/10.1006/jmra.1994.1008
Fan T (1996) Metabolite profiling by one- and two-dimensional NMR analysis of complex mixtures. Prog Nucl Mag Reson Spectrosc. 28:161–219
Govindaraju V, Young K, Maudsley AA (2000) Proton NMR chemical shifts and coupling constants for brain metabolites. NMR Biomed. 13(3):129–153
Gasparovic C, Song T, Devier D, Bockholt HJ, Caprihan A, Mullins PG et al (2006) Use of tissue water as a concentration reference for proton spectroscopic imaging. Magn Reson Med. 55(6):1219–1226
Mlynárik V, Gruber S, Moser E (2001) Proton T (1) and T (2) relaxation times of human brain metabolites at 3 Tesla. NMR Biomed. 14(5):325–331
Zhang Y, Brady M, Smith S (2001) Segmentation of brain MR images through a hidden Markov random field model and the expectation-maximization algorithm. IEEE Trans Med Imaging. 20(1):45–57
Cavassila S, Deval S, Huegen C, van Ormondt D, Graveron-Demilly D (2001) Cramér-Rao bounds: an evaluation tool for quantitation. NMR Biomed. 14(4):278–283
Laitinen J, Samarut J, Hölttä E (1994) A nontoxic and versatile protein salting-out method for isolation of DNA. Biotechniques 17(2):316–322
Benes FM, Berretta S (2001) GABAergic interneurons: implications for understanding schizophrenia and bipolar disorder. Neuropsychopharmacology. 25(1):1–27
Brix MK, Ersland L, Hugdahl K, Grüner R, Posserud M-B, Hammar Å et al (2015) Brain MR spectroscopy in autism spectrum disorder-the GABA excitatory/inhibitory imbalance theory revisited. Front Hum Neurosci. 9:365
Bush G, Luu P, Posner M (2000) Cognitive and emotional influences in anterior cingulate cortex. Trends Cogn Sci (Regul Ed). 4(6):215–222
Lewis DA, Levitt P (2002) Schizophrenia as a disorder of neurodevelopment. Annu Rev Neurosci. 25:409–432
Hibar DP, Westlye LT, Doan NT, Jahanshad N, Cheung JW, Ching CRK et al (2018) Cortical abnormalities in bipolar disorder: an MRI analysis of 6503 individuals from the ENIGMA Bipolar Disorder Working Group. Mol Psychiatry 23(4):932–942. https://doi.org/10.1038/mp.2017.73
Sanacora G, Mason GF, Rothman DL, Krystal JH (2002) Increased occipital cortex GABA concentrations in depressed patients after therapy with selective serotonin reuptake inhibitors. Am J Psychiatry. 159(4):663–665
Stahl SM (2009) The Prescriber's Guide, antipsychotics and mood stabilizers. Cambridge University Press, Cambridge
Cunningham MO, Dhillon A, Wood SJ, Jones RS (2000) Reciprocal modulation of glutamate and GABA release may underlie the anticonvulsant effect of phenytoin. Neuroscience 95(2):343–351
Friedman SD, Dager SR, Parow A, Hirashima F, Demopulos C, Stoll AL et al (2004) Lithium and valproic acid treatment effects on brain chemistry in bipolar disorder. Biol Psychiatry. 56(5):340–348
Scarr E, Pavey G, Sundram S, MacKinnon A, Dean B (2003) Decreased hippocampal NMDA, but not kainate or AMPA receptors in bipolar disorder. Bipolar Disord. 5(4):257–264
Beneyto M, Kristiansen LV, Oni-Orisan A, McCullumsmith RE, Meador-Woodruff JH (2007) Abnormal glutamate receptor expression in the medial temporal lobe in schizophrenia and mood disorders. Neuropsychopharmacology. 32(9):1888–1902
Goto N, Yoshimura R, Kakeda S, Nishimura J, Moriya J, Hayashi K et al (2012) Six-month treatment with atypical antipsychotic drugs decreased frontal-lobe levels of glutamate plus glutamine in early-stage first-episode schizophrenia. Neuropsychiatr Dis Treat. 8:119–122
Luo J, Min S, Wei K, Li P, Dong J, Liu Y-F (2011) Propofol protects against impairment of learning-memory and imbalance of hippocampal Glu/GABA induced by electroconvulsive shock in depressed rats. J Anesth. 25(5):657–665
Sanacora G, Mason GF, Rothman DL, Hyder F, Ciarcia JJ, Ostroff RB et al (2003) Increased cortical GABA concentrations in depressed patients receiving ECT. Am J Psychiatry. 160(3):577–579
Kessing LV (2019) What is early intervention in bipolar disorder? Recommendation of a pragmatic way focusing on early intervention in patients with newly diagnosed bipolar disorder. Bipolar Disord 21(2):168–169. https://doi.org/10.1111/bdi.12733
Kennedy SH, Lam RW, McIntyre RS, Tourjman SV, Bhat V, Blier P et al (2016) Canadian network for mood and anxiety treatments (CANMAT) 2016 clinical guidelines for the management of adults with major Depressive Disorder: Section 3. Pharmacological Treatments. Can J Psychiatry. 61(9):540–560
Acknowledgements
We would like to thank the members of the Mood Disorders Unit (GRUDA) for their work, as well as the volunteers for their collaboration.
Funding
The Sao Paulo Research Foundation (FAPESP) financed this study (2012/23796-2 and 2010/12286-8).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
None of the authors report biomedical financial interests or potential conflicts of interest.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Scotti-Muzzi, E., Chile, T., Moreno, R. et al. ACC Glu/GABA ratio is decreased in euthymic bipolar disorder I patients: possible in vivo neurometabolite explanation for mood stabilization. Eur Arch Psychiatry Clin Neurosci 271, 537–547 (2021). https://doi.org/10.1007/s00406-020-01096-0
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00406-020-01096-0